Abstract
Efficient cryopreservation conditions for the edible alkalophilic cyanobacterium Arthrospira (Spirulina) platensis were investigated using a model strain A. platensis NIES-39. As a result, it was found that more than 60% of cells were viable upon thawing, when they had been frozen at a cooling rate of approximately −1 °C min−1 in the presence of 10% (v/v) dimethyl sulfoxide. Further examination with other Arthrospira strains showed that many of them had strain-dependent optimal conditions for cryopreservation. For example, the best freezing conditions for A. platensis SAG 21.99 were snap-freezing in liquid nitrogen in the presence of 5% (v/v) dimethyl sulfoxide, while they were slow cooling at approximately −1 °C min−1 in the presence of 10% (v/v) methanol for A. platensis NIES-46, NIES-2308 and UTEX 1926. The variety of successful cryopreservation conditions presented in this study is useful when attempting to cryopreserve various Arthrospira strains.
Graphical abstract
The conditions for efficient cryopreservation of Arthrospira platensis were determined. They enable genetically stable preservation of many A. platensis strains.

The filamentous cyanobacteria Arthrospira platensis and A. maxima have been consumed as food by people living along alkaline lakes in several regions of the world.Citation1–3) They are rich in protein, minerals, and vitamins and also contain other beneficial metabolites, such as β-carotene and γ-linolenic acid.Citation4–6) Because Arthrospira species grow under alkaline conditions that suppress the propagation of other microalgae, they can be propagated relatively easily as unialgal cultures in open ponds. This feature is suitable for large-scale commercial propagation. Currently, these cyanobacteria are industrially cultured in many tropical and subtropical regions and are consumed worldwide as a nutrient source and food additives.Citation1,2) Their products are marketed under the name spirulina, as they had formerly been classified in the genus Spirulina.
In addition to their industrial use, the use of Arthrospira has also been attempted to combat global malnutrition, particularly in developing countries.Citation7–10) Because Arthrospira can be cultured relatively easily in open ponds and harvested by simple filtration, it can be produced and harvested by local people for daily consumption using non-arable land. Its high nutritional value, especially its high protein content (50–70% of biomass in dry weight),Citation4,5) is expected to help improve the nutritional status of otherwise undernourished people. A recent study on the effect of spirulina on malnourished children showed that a daily intake of 10 g of A. platensis significantly improved the height of stunted children, proving that it is useful to alleviate problems caused by a poor or inadequate staple diet.Citation10)
A number of wild-type Arthrospira strains have been collected from many different parts of the world.Citation1,3) They are slightly different from each other in their morphological, physiological, and biochemical properties.Citation3, 11–14) Many of them are currently maintained by regular sub-culturing, rather than by cryopreservation, because reliable cryopreservation methods do not exist. This may cause an eventual loss of their valuable traits through the accumulation of spontaneous mutations. Previous attempts to cryopreserve Arthrospira strains suggested that commonly used cryopreservation conditions for cyanobacteria were unsuitable for them. For example, when 144 cyanobacterial strains (52 species), including two A. platensis strains, were frozen by a conventional two-step cooling method, survival of a significant number of cells was detected for as many as 136 strains (49 species). However, neither of the two A. platensis strains was cryopreserved efficiently by the employed method.Citation15) Other studies also demonstrated that the cryopreservation efficiency of A. platensis strains was unacceptably low; viable cells could be recovered from either only <5% of the sample vialsCitation16) or none of themCitation17) after cryopreservation.
In the present study, the cryopreservation efficiency of A. platensis NIES-39, one of the model Arthrospira strains whose complete genome sequence had been determined,Citation18) was examined under various conditions. As a result, it was found to be efficiently cryopreserved under certain conditions. The cryopreservation efficiency of six other Arthrospira strains was also examined under various conditions. The experimental results indicated that a considerable proportion of Arthrospira strains could be cryopreserved fairly efficiently when they were frozen under strain-dependent optimal conditions.
Materials and methods
Strains and culture conditions
A. platensis NIES-39, A. platensis NIES-46 and A. platensis NIES-2308 were obtained from the Microbial Culture Collection at the National Institute for Environmental Studies, Tsukuba (MCC-NIES). A. platensis UTEX 1926 was obtained from the Culture Collection of Algae at the University of Texas at Austin (UTEX). A. platensis SAG 21.99, A. platensis SAG 85.79 and A. maxima SAG 84.79 were obtained from Sammlung von Algenkulturen der Universität Göttingen (SAG). Arthrospira strains were cultured at 30 °C under a 12:12 h light:dark cycle in SOT mediumCitation19) that was prepared as previously described,Citation20) except that the medium used for A. platensis UTEX 1926 was 0.5 × SOT medium.Citation14)
Determination of trichome concentrations in cultures
After 2–20 μL aliquots of cultures were spotted on half-dried agar plates, the number of trichomes was visually counted under a dissecting microscope. Trichomes shorter than approximately 100 μm, which are present in many Arthrospira cultures as a minor population, were omitted from the count since they did not significantly contribute to the total biomass. The trichome concentration was calculated by dividing the number of trichomes by volume. When the trichome concentration was too high and hampered a visual count, cultures were diluted beforehand with SOT medium. Small volumes of cultures were placed on agar plates by automatic micropipettes (Pipetman, Gilson Inc., Middleton) with disposable polypropylene tips for all strains, except A. platensis NIES-2308. For A. platensis NIES-2308, disposable glass micropipettes (Calibrated Micropipets, Drummond Scientific Co., Broomall) were used because the trichomes of this strain tended to be adsorbed by the internal surface of the polypropylene tips.
Determination of recovery rates after freezing and thawing
The recovery rate, which was arbitrarily defined in this study, provides a rough estimate of the probability of recovering a considerable number of viable cells after the freezing of 1 × 104 trichomes. This was determined as follows. Arthrospira trichomes in the late-log phase were collected with filtration tubes.Citation14) Then, they were suspended in SOT medium containing various concentrations of glycerol, dimethyl sulfoxide (DMSO), or methanol (MeOH) to make trichome suspensions of approximately 2 × 105 trichomes ml−1 (the OD730 values of these suspensions were approximately 0.85). From each suspension, 50 μl aliquots (approximately 1 × 104 trichomes) were dispensed into 8–16 microfuge tubes. These were left for 15 min at room temperature and then either snap-frozen by putting them in liquid nitrogen or frozen at a cooling rate of approximately −1 °C min−1 using isopropyl alcohol-insulated cryopreservation containers (Nalgene Mr. Frosty™ Cryo 1 °C Freezing Container, Nalge Nunc International Corp., New York). Samples in cryopreservation containers that had initially been kept at room temperature (approximately 25 °C) were cooled by putting the containers in a −80 °C freezer for 24 h. Samples snap-frozen in liquid nitrogen were transferred to a −80 °C freezer and stored for 24 h. After 24 h, samples were thawed rapidly by adding 950 μl of SOT medium that had been pre-warmed to 37 °C, followed by incubation at 37 °C for approximately 1 min with occasional mixing. The thawed samples were then cultured at 30 °C under standard conditions. After four days, the optical density at 730 nm (OD730) of each sample was determined with a spectrophotometer (Novaspec II, Pharmacia-LKB Biotechnology, Uppsala), and the ratio of the number of samples with an OD730 > 0.15 to the total number of samples were determined for each freezing condition. The obtained values were multiplied by 100 to obtain recovery rates as a percentage. For each freezing condition, experiments were repeated 8–10 times to draw a box plot.
Determination of survival rates after freezing and thawing
The survival rate, as determined below, provides a rough estimate of the ratio of cells that survived the freezing and thawing process. Freezing and thawing of samples were carried out in the same way as that used to determine the recovery rate. After samples were thawed, they were cultured under standard conditions. The OD730 of the frozen-and-thawed samples and unfrozen controls was determined when the control samples were growing exponentially (typically four days after the beginning of culture). Survival rates were determined by dividing the OD730 values of the frozen-and-thawed samples by those of the unfrozen controls that had been cultured for the same period of time. This value gives a rough estimate of the ratio of cells that survived the freezing and thawing process. However, when sample OD730 values were too small, accurate survival rates could not be determined by this method, because background light scattering by broken cell debris was significant. Therefore, when OD730 values of any sample was <0.1, survival rates were determined by counting the actual numbers of trichomes under a dissecting microscope. In this case, the number of trichomes in the cultures of frozen-and-thawed samples was divided by the number of trichomes in the control culture that had been grown for the same period of time. The obtained values were multiplied by 100 to obtain survival rates as a percentage.
Results
Effect of different freezing conditions on the recovery of viable cells
To determine efficient cryopreservation conditions, A. platensis NIES-39 was frozen in the presence of various concentrations of glycerol, DMSO and MeOH. Two different cooling methods were employed in the experiment: (1) snap-freezing in liquid nitrogen and (2) slow cooling in isopropyl alcohol-insulated cryopreservation containers that allowed cooling at a rate of approximately −1 °C min−1 when placed in a −80 °C freezer.Citation21) Although this kind of passive freezing in isopropyl alcohol-insulated containers has been widely used in the cryopreservation of various cells,Citation22,23) there are no reports on their use to cryopreserve Arthrospira strains.
To elucidate the cryopreservation efficiency of bacteria, survival rates are usually determined from the difference of colony-forming units (cfu) before and after cryopreservation. Unfortunately, most A. platensis strains do not form colonies on a solid medium because they are active in a gliding movement.Citation12) Therefore, to compare the survival of Arthrospira cells after cryopreservation, the following standardized procedure that did not rely on the determination of cfu was employed. In that procedure, 50 μL aliquots of trichome suspensions each containing approximately 1 × 104 trichomes were frozen under various conditions. After storage at −80 °C, samples were rapidly thawed by adding an excess volume (950 μL) of growth medium that had been pre-warmed to 37 °C. The thawed samples were cultured, and OD730 was determined after four days. The ratio of samples that grew to OD730 of more than 0.15 in four days was recorded for each set of conditions, and this was designated as the recovery rate. The initial OD730 value of the samples prepared as above was approximately 0.067, when they had not been frozen. Therefore, if the OD730 value increased to >0.15 after the culture period, it means that the number of cells increased to more than double as compared to the initial number of cells, indicating that a considerable proportion of cells revived and propagated after thawing.
Fig. shows examples of samples that were frozen under various conditions and cultured for four days after thawing. The OD730 values of unfrozen controls were on average 0.067 at the beginning, and they were approximately 0.4 on the fourth day (Fig. (A)). The cell concentrations of the frozen-and-thawed samples after the culture period were variable depending on the freezing conditions (Fig. (B)).
Fig. 1. Cultures of A. platensis NIES-39 after freezing and thawing.
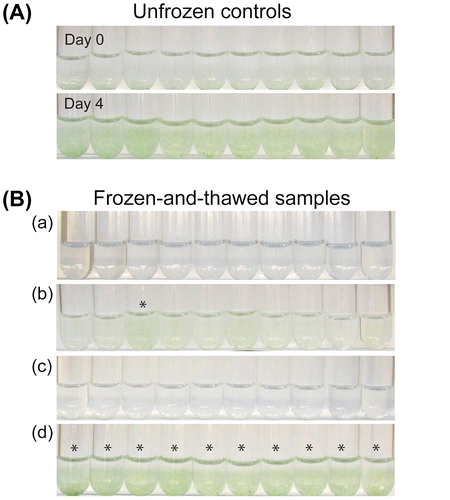
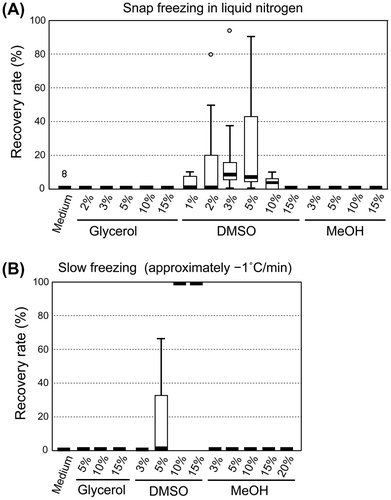
Fig. shows the recovery rate distributions after freezing A. platensis NIES-39 cells under various conditions. As shown, glycerol and MeOH were not effective cryoprotectants under all conditions tested. In contrast, when DMSO was used, the recovery of viable cells was observed under various conditions. The combination of slow freezing (approximately −1 °C min−1) and a relatively high concentration of DMSO (10–15%) was particularly effective with a constant 100% recovery rate associated with this combination (Fig. ).
Fig. 2. Recovery rate of A. platensis NIES-39 frozen under various conditions.
Growth of A. platensis NIES-39 after thawing
Growth curves were determined to examine the growth of A. platensis NIES-39 after cryopreservation. As shown in Fig. , the OD of the cultures rapidly decreased when inappropriate conditions (e.g. snap-freezing in the presence of 5% glycerol) were used for freezing. The decreased OD did not increase afterwards. In contrast, when cells had been frozen at approximately −1 °C min−1 in the presence of 10% (v/v) DMSO, a rapid recovery of cell growth was observed. Comparison of the growth curve of the frozen-and-thawed sample with that of the unfrozen control indicated that more than 60% of cells survived the freezing-and-thawing process when cells had been frozen under these optimal conditions. Cell growth was also observed when cells were snap-frozen in the presence of 5% (v/v) DMSO. However, in this case, the ratio of survived cells appeared to be much lower than that frozen under optimal conditions, resulting in a delay in the increase of OD (Fig. ).
Fig. 3. Growth of A. platensis NIES-39 after freezing and thawing.
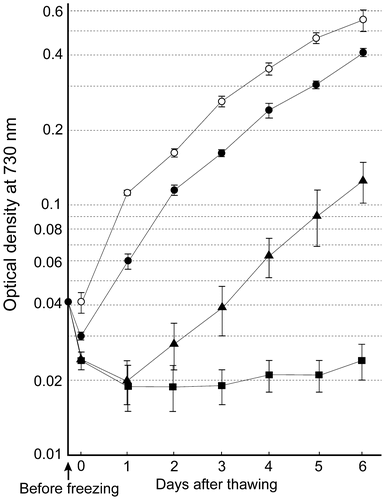
This experiment showed that comparisons of the growth curve of frozen-and-thawed samples with that of unfrozen controls is helpful to quantitatively estimate cell survival rates. For example, on day three in Fig. , the ratio of the ODs of the samples that had been frozen at approximately −1 °C min−1 in the presence of 10% DMSO to those of unfrozen controls averaged 0.68. Therefore, it was estimated that an average of 68% of cells survived the freezing and thawing process under these conditions. Since the survival rates determined in this way were useful to estimate the ratio of survived cells, it was used to compare the results of various experiments in the following sections.
Recovery of A. platensis NIES-39 after prolonged storage
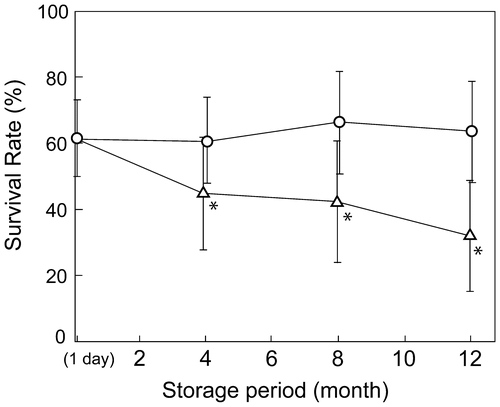
As noted above, A. platensis NIES-39 were efficiently cryopreserved when they had been frozen at a cooling rate of approximately −1 °C min−1 in the presence of 10% (v/v) DMSO. To determine whether viable cells could be recovered after prolonged storage, cells were stored either in a −80 °C freezer or in the vapor phase of liquid nitrogen for up to one year. As shown in Fig. , there was no detectable decrease in the survival rate when they were stored in the liquid nitrogen vapor phase, while there was an approximately 50% decrease after one-year storage at −80 °C. These results indicated that storage in the liquid nitrogen vapor phase was preferable for long-term storage, while storage at −80 °C might be permissive for mid-term storage (e.g. storage for up to six months) for applications that do not require the highest cryopreservation efficiency.
Fig. 4. Survival rate of A. platensis NIES-39 after prolonged cryopreservation.
Survival rate of various Arthrospira strains after cryopreservation
To test whether the optimal conditions for A. platensis NIES-39 were also effective for other Arthrospira strains, cryopreservation efficiency of six other strains (A. platensis NIES-46, A. platensis NIES-2308, A. platensis UTEX 1926, A. platensis SAG 21.99, A. platensis SAG 85.79, and A. maxima SAG 84.79) was examined after freezing under the same conditions. However, their cryopreservation efficiencies were considerably lower than that of A. platensis NIES-39. Since it was possible that they had different optimal condition preferences, cryopreservation efficiency was examined under various freezing conditions for each strain. Since it was observed in preliminary experiments that glycerol was not an effective cryoprotectant for any of the strains, only the effects of DMSO and MeOH were examined in the following quantitative experiments.
Survival rates of the strains frozen under various conditions are shown in Table (results of control experiments performed without cryoprotectants are presented in Supplemental Table S1). As shown in Table , most strains had strain-dependent preferable conditions. For example, A. platensis NIES-46, A. platensis NIES-2308, and A. platensis UTEX 1926 were more efficiently cryopreserved when they were frozen at a cooling rate of approximately −1 °C min−1 in the presence of 10% MeOH; these conditions were unsuitable for A. platensis NIES-39. On the other hand, A. platensis SAG 21.99 was cryopreserved efficiently when it was snap-frozen in liquid nitrogen in the presence of 5% (v/v) DMSO. Similarly, the preferred condition of A. platensis SAG 85.79 and A. maxima SAG 84.79 was snap-freezing in liquid nitrogen in the presence of 5% (v/v) DMSO, although their survival rates were, at best, much lower than those of other strains that were frozen under optimal conditions.
Table 1. Survival rate of various Arthrospira strains after freezing and thawing.
Survival rates of A. platensis SAG 85.79 and A. maxima SAG 84.79 were further examined under other conditions (Supplemental Table S2). The survival rate of A. platensis SAG 85.79 was slightly improved when this strain was snap-frozen in the presence of 10% (v/v) DMSO, but it was still considerably low (1.31% on average). For A. maxima SAG 84.79, no cell survival was detected under any of the newly set conditions (Supplemental Table S2).
Discussion
Many studies have suggested that, among cyanobacteria, Arthrospira strains are particularly difficult to cryopreserve.Citation15–17) However, experiments with A. platensis NIES-39 in this study indicated that this strain could be cryopreserved efficiently when it was frozen at a cooling rate of approximately −1 °C min−1 in the presence of 10% (v/v) DMSO (Fig. and Table ). Although a combination of these conditions was not employed in any published studies to cryopreserve Arthrospira strains, the results of the present study indicate that this combination is worth pursuing when attempting to cryopreserve various Arthrospira strains.
Examinations of other Arthrospira strains indicated that they had strain-dependent optimal conditions for cryopreservation (Table ). Slow freezing in the presence of 10% (v/v) MeOH was preferable for A. platensis NIES-46, A. platensis NIES-2308 and A. platensis UTEX 1926. Because a considerable proportion of strains used in this study were cryopreserved with fair efficiencies under these conditions, this is also worth employing when attempting to cryopreserve various Arthrospira strains.
The cryopreservation efficiency of A. platensis SAG 21.99 was low when it was slowly frozen, but it was cryopreserved more efficiently with an average survival rate of 81.7% when it was snap-frozen in liquid nitrogen in the presence of 5% (v/v) DMSO (Table ). Similar conditions were employed in a former attempt to cryopreserve Arthrospira strains, but results were negative for all tested strains.Citation17) However, our experimental results with A. platensis SAG 21.99, which was not included in the former attempt,Citation17) suggest the possibility that there are still a considerable number of strains that can be cryopreserved efficiently under these conditions. Therefore, when attempting to cryopreserve various Arthrospira strains, snap-freezing in liquid nitrogen would also be warranted as a potential cooling method.
Arthrospira strains had strain-dependent optimal conditions for cryopreservation. There was no single set of conditions that could be used to cryopreserve all Arthrospira strains. When attempting to cryopreserve various Arthrospira strains whose optimal cryopreservation conditions are unknown, it is advisable to use the following three sets of conditions: (1) freezing at a cooling rate of approximately −1 °C min−1 in the presence of 10% (v/v) DMSO, (2) freezing at a cooling rate of approximately −1 °C min−1 in the presence of 10% (v/v) MeOH and (3) snap-freezing in liquid nitrogen in the presence of 5% (v/v) DMSO. If these sets of conditions had been used to cryopreserve the seven strains employed in this study, five strains (71.4%) would have been cryopreserved with fair efficiency with the least average survival rate of 26.7% for A. platensis NIES-2308 (Table ).
Although many of the strains were cryopreserved fairly efficiently under appropriate conditions, A. platensis SAG 85.79 and A. maxima SAG 84.79 were not cryopreserved efficiently under any of the conditions employed in this study (Table and Supplemental Table 2). However, it is worth noting that only a small number of viable trichomes could be recovered for each of these strains after snap-freezing in the presence of an appropriate concentration of DMSO (Table ), and they could be propagated to obtain dense cultures. However, it would be too risky to rely on such a low-efficiency cryopreservation method when storing these strains. It is expected that there are a considerable number of strains, like these strains, that are not eligible for cryopreservation, even if the above-mentioned sets of conditions are employed. Efficient cryopreservation conditions for such strains remain to be investigated.
To obtain reproducible results in a series of cryopreservation experiments, it was important to keep frozen samples at below-freezing temperature until they were rapidly thawed. Even placing frozen samples at an ambient temperature for 90 s before thawing reduced the survival rate. In the present study, when samples were transferred from a freezer or a liquid nitrogen container to a lab bench where sample thawing was carried out, they were always placed in an isopropanol-insulated cryopreservation container that had been cooled beforehand in a −80 °C freezer. In addition to keeping samples at low temperature before thawing, rapid thawing was also important for obtaining reproducible results. In the present study, samples were thawed by adding an excess volume of warmed (37 °C) growth medium. When the growth medium added at this step was at ambient temperature, a reduction in the survival rate was observed. This care before and during sample thawing may explain the cell survival of many strains, even after snap-freezing in liquid nitrogen in the presence of 5% DMSO (Table ). Under similar freezing conditions, no cell survival was observed for any strains.Citation17)
In this study, A. platensis NIES-39 was initially employed to examine cryopreservation efficiency under various freezing conditions. The reason that this strain was chosen was because it was one of the potential model Arthrospira strains whose complete genome sequence had been determined.Citation18) Although it was not expected beforehand, subsequent experiments with other Arthrospira strains revealed that this strain was particularly tolerant to freezing in the presence of various DMSO concentrations (Table ). In molecular biological studies of micro-organisms, it is often necessary to handle a number of variant strains (e.g. mutants and transformants). To be used in such studies, successful cryopreservation is a great advantage for a starting strain, because it allows the preservation of many variant strains without the cumbersome maintenance by sub-culturing. Therefore, this study suggests that A. platensis NIES-39 is potentially suitable as a model strain for the molecular biological study of A. platensis.
In conclusion, this study has shown that a considerable proportion of Arthrospira strains can be cryopreserved fairly efficiently. The window of freezing conditions provided in this study should be useful when attempting to cryopreserve various Arthrospira strains.
Disclosure statement
No potential conflict of interest was reported by the author.
Funding
This work was supported by the Grant-in-Aid for Challenging Exploratory Research from the Japan Society for the Promotion of Science (JSPS KAKENHI) [grant number 26660063]; Management Expenses Grants for National University Corporations from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Supplementary material
The supplementary material for this paper is available online at http://dx.doi.org/10.1080/09168451.2016.1189320
TBBB_1189320_Supplementary_Material.docx
Download MS Word (17.5 KB)Acknowledgments
The author would like to thank Enago (www.enago.jp) for the English language review.
Notes
Abbreviations: DMSO, dimethyl sulfoxide; MeOH, methanol; cfu, colony forming unit.
References
- Belay A. Biology and industrial production of Arthrospira (Spirulina). In: Richmond A, Hu Q, editors. Handbook of microalgal culture. 2nd ed. West Sussex: Wiley-Blackwell; 2013. p. 339–358.10.1002/9781118567166
- Grewe CB, Pulz O. The biotechnology of cyanobacteria. In: Whitton BA, editor. Ecology of cyanobacteria II: their diversity in space and time. Dordrecht: Springer; 2012. p. 707–739.10.1007/978-94-007-3855-3
- Sili C, Torzillo G, Vonshak A. Arthrospira (Spirulina). In: Whitton BA, editor. Ecology of cyanobacteria II: their diversity in space and time. Dordrecht: Springer; 2012. p. 677–705.10.1007/978-94-007-3855-3
- Devi M, Subbulakshmi G, Devi K, et al. Studies on the proteins of mass-cultivated, blue-green alga (Spirulina platensis). J. Agric. Food Chem. 1981;29:522–525.10.1021/jf00105a022
- Narasimha DLR, Venkataraman GS, Duggal SK, et al. Nutritional quality of the blue-green alga Spirulina platensis Geitler. J. Sci. Food Agric. 1982;33:456–460.10.1002/(ISSN)1097-0010
- Cohen Z, Vonshak A, Richmond A. Fatty acid composition of Spirulina strains grown under various environmental conditions. Phytochemistry. 1987;26:2255–2258.10.1016/S0031-9422(00)84694-4
- Jeeji Bai N, Seshadri CV. Small scale culture of Spirulina (Arthrospira) as food supplement for rural households – technology development and transfer. Algol. Stud. 1988;50–53, 565–572.
- Simpore J, Kabore F, Zongo F, et al. Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nut. J. 2006;5:3.10.1186/1475-2891-5-3
- Habib MAB, Pariv M, Huntington TC, et al. A review on culture, production and use of spirulina as food for humans and feeds for domestic animals and fish (FAO Fisheries and Aquaculture Circular No. 1034). Rome: Food and Agriculture Organisation of the United Nations; 2008.
- Masuda K, Inoue Y, Inoue R, et al. Spirulina effectiveness study on child malnutrition in Zambia. In: Harris J, Haddad L, Grütz SS, editors. Turning rapid growth into meaningful growth: sustaining the commitment to nutrition in Zambia. Brighton: Institute of Development Studies; 2014. p. 49–56.
- Mühling M, Belay A, Whitton BA. Variation in fatty acid composition of Arthrospira (Spirulina) strains. J. Appl. Phycol. 2005;17:137–146.10.1007/s10811-005-7213-9
- Mühling M, Somerfield PJ, Harris N, et al. Phenotypic analysis of Arthrospira (Spirulina) strains (cyanobacteria). Phycologia. 2006;45:148–157.10.2216/05-21.1
- Satora P, Barwińska-Sendra A, Duda-Chodak A, et al. Strain-dependent production of selected bioactive compounds by Cyanobacteria belonging to the Arthrospira genus. J. Appl. Microbiol. 2015;119:736–743.10.1111/jam.12897
- Shiraishi H. Association of heterotrophic bacteria with aggregated Arthrospira platensis exopolysaccharides: implications in the induction of axenic cultures. Biosci. Biotechnol. Biochem. 2015;79:331–341.10.1080/09168451.2014.972333
- Mori F, Erata M, Watanabe MM. Cryopreservation of cyanobacteria and green algae in the NIES-Collection. Microbiol. Cult. Coll. 2002;18:45–55.
- Motham M, Peerapornpisal Y, Tongsriri S, et al. High subzero temperature preservation of Spirulina platensis (Spirulina fusiformis) and its ultrastructure. Chiang Mai J. Sci. 2012;39:554–561.
- Mühling M. Characterization of Arthrospira (Spirulina) strains [dissertation]. Durham E-Theses Online edition. Durham: Durham University; 2000. Available from: http://etheses.dur.ac.uk/1198/
- Fujisawa T, Narikawa R, Okamoto S, et al. Genomic structure of an economically important cyanobacterium, Arthrospira platensis NIES-39. DNA Res. 2010;17:85–103.10.1093/dnares/dsq004
- Ogawa T, Terui G. Studies on the growth of Spirulina platensis. (I) On the pure culture of Spirulina platensis. J. Ferment Technol. 1970;48:361–367.
- Shiraishi H, Tabuse Y. The AplI restriction-modification system in an edible cyanobacterium, Arthrospira (Spirulina) platensis NIES-39, recognizes the nucleotide sequence 5′-CTGCAG-3′. Biosci. Biotechnol. Biochem. 2013;77:782–788.10.1271/bbb.120919
- Buhl T, Legler TJ, Rosenberger A, et al. Controlled-rate freezer cryopreservation of highly concentrated peripheral blood mononuclear cells results in higher cell yields and superior autologous T-cell stimulation for dendritic cell-based immunotherapy. Cancer Immunol. Immunother. 2012;61:2021–2031.10.1007/s00262-012-1262-0
- Day JG, Brand JJ. Cryopreservation methods for maintaining microalgal cultures. In: Andersen RA, editor. Algal Culturing Techniques. Burlington, VT: Elsevier Academic Press; 2005. p. 165–187.
- Stacey GN, Masters JR. Cryopreservation and banking of mammalian cell lines. Nat. Protoc. 2008;3:1981–1989.10.1038/nprot.2008.190
