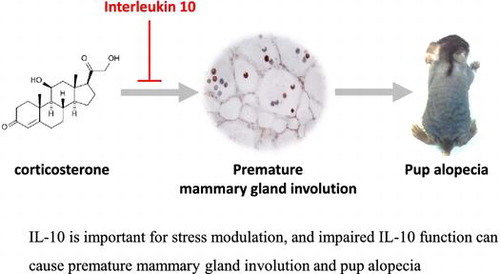
Graphical abstract
IL-10 is important for stress modulation, and impaired IL-10 function can cause premature mammary gland involution and pup alopecia.
Abstract
Recently, we found that maternal stress could induce premature mammary gland involution in interleukin 10 knock out (IL-10−/−) mice. To elucidate correlation between stress, IL-10, and mammary gland involution, corticosterone was injected into the lactating wild type and IL-10-deficient mice and assessed mammary gland phenotype. Repetitive corticosterone injection developed premature mammary gland involution only in B6.IL-10−/− mice; moreover, it induced alopecia in nursing pups. Corticosterone injection induced several typical changes such as mammary gland epithelial cell apoptosis, macrophage infiltration, fat deposition in adipocyte, STAT3 phosphorylation, and upregulation of tyrosine hydroxylase gene in adrenal gland. Overall incidence of pup alopecia and mammary gland involution was relatively high in corticosterone than control B6.IL-10−/− group (57% vs. 20%). Our finding demonstrates that IL-10 is important for stress modulation, and B6.Il-10−/− with corticosterone has several advantage such as simple to establish, well-defined onset of mammary gland involution, high incidence, and inducing pup alopecia.
Key words:
Mammary gland involution is the process that mammary glands recover to a non-lactating state after weaning and is characterized by epithelial cell death and tissue remodeling. This process is important for initialization of mammary gland function, and it is multistage process. In the first stage, milk accumulation stress induces mammary gland epithelial cell apoptosis, which causes apoptotic alveolar cells to detach and shed into lumen. In the second stage, apoptosis is widely observed and followed by remodeling of the surrounding stroma and re-differentiation of adipocytes.Citation1,2)
During milk stasis period, several genes such as TGFb3,Citation3) LIF, and oncostatin-MCitation4) were activated and induced Stat3 activation. In addition, TGFb3,Citation3) LIFCitation5), and IL-6Citation6) were involved in Stat3 activation in first stage. Signal transducer and activator of transcription 3 (Stat3) control apoptosis during mammary gland involution by regulation in lysosomal-mediated programmed cell deathCitation7) and target genes of phosphorylated Stat3 are important for early apoptosis. Phagocytosis is important for the removal of apoptotic cells and debris; the influx of macrophages is seen at day 4 of mammary gland involution.Citation8)
IL-10 is an important anti-inflammatory cytokine, but a few study was conducted about role of IL-10 in mammary gland involution. It was reported that IL-10 participates in mammary gland apoptosis through a specific death pathway in the mammary gland, and IL-10 only induces a mild infiltration by lymphocytes.Citation9) Recently, we reported that maternal stress such pregnancy in postpartum estrus induces untimely mammary gland involution and inflammatory milk production in interleukin 10 (IL-10)-deficient mice.Citation10) This result might indicate that IL-10 is important for regulating premature mammary gland involution. Furthermore, there were several previous studies about premature mammary gland involution relating plasminogenCitation11) about delayed mammary gland involution with EZH2 gene,Citation12) and impaired mammary gland involution relating milk fat globule EGF factor 8.Citation13)
We hypothesize that IL-10 is important for regulating stress responses and has role for preventing premature mammary gland involution. To elucidate correlation between stress, IL-10, and mammary gland involution, corticosterone was injected into the lactating wild type and IL-10-deficient mice and assessed mammary gland phenotype.
Materials and methods
Animal experiment
C57BL/6J (B6) and C57BL/6.129P2-Il10tm1Cgn/J (B6.IL-10−/−) were purchased from the Jackson laboratory. All mice were reared in individual ventilated cage rack with ad libitum access to feed and water and maintained specific pathogen-free status. Twelve multiparous B6.IL-10−/− and 8 B6 dams were selected, and monogamous mating was conducted without using the postpartum estrus. Before parturition, male mouse was removed from the mating cage, and each dam was maintained solely during nursing. After delivery, number of pups of each dam was synchronized to 7. Dams were randomly divided into 2 groups and were administered with corticosterone (Sigma-Aldrich, Saint Louis, MO, USA) and vehicle via subcutaneous route as previously reported, with minor modifications.Citation14) In brief, corticosterone stock was prepared by dissolving 50 mg of corticosterone in 75 μl dimethyl sulfoxide. Phosphate-buffered saline with 0.1% Tween-80 was used as vehicle. Twenty milligrams of corticosterone with 180 μl of vehicle was administered to the corticosterone group, and same volume of vehicle was injected to control group. Daily corticosterone and vehicle injection was performed from postnatal day 4 (P4) to P17. During P4 to P17, weight measurement was conducted for all pups from corticosterone and control mice.
Dam and pups were euthanized by cervical dislocation at P19, and sampling was conducted in same time to minimize influence of circadian rhythm. Mammary gland from dams and skin from pups were fixed formalin and optimum cutting temperature compound medium (Sakura Finetek, Torrance, CA). Adrenal gland tissues and serums from dams were collected and stored in −70 °C until use. This study and animal use protocol were approved by the Institutional Animal Care and Use Committee of Seoul National University.
Histological examination
Hematoxylin and eosin (H&E) stain, immunohistochemistry, TUNEL stain, and oil red O (ORO) stain were conducted on 5-μm sections. To analyze apoptosis of mammary gland, number of apoptotic bodies from 6 to 7 regions (600 μm × 670 μm) were calculated and compared.
In immunochemistry, formalin-fixed and frozen tissue was conducted , and perilipin (Cell Signaling Technology, Beverly, MA, USA), F4/80 (eBioscience, San Diego, CA, USA), and keratin 18 (Santa Cruz, Dallas, TX, USA) were used as primary antibodies. 3,3′-Diaminobenzidine (DAB, Vector Laboratories Inc., Burlingame, CA, USA) was used as a substrate. Detail information was described in Table .
Table 1. Used antibodies for immunohistochemistry.
TUNEL stain was conducted with ApopTag peroxidase in situ Apoptosis Detection Kit (EMD Millipore, Billerica, MA, USA) as per the manufacturer’s instruction with formalin-fixed mammary gland tissue sections and DAB (Vector) as substrate. ORO staining was conducted using the frozen mammary gland tissue of 5-μm section. After air-drying at room temperature, the section was fixed with 10% formalin for 10 min and rinsed with isopropanol. This was followed by staining using ORO working solution (Sigma) and counter staining.Citation15)
Quantitative mRNA expression analysis for tyrosine hydroxylase gene
Quantitative RT-PCR with SYBR was conducted using ABI 7500 (Applied Biosystems, Foster City, CA, USA). The cDNAs were prepared using adrenal gland tissue from corticosterone- and vehicle-injected dams. For amplification of tyrosine hydroxylase (TH) gene, the forward primer as 5′-GGAGTTTGGGCTGTGTAA-3′ and the reverse primer sequence as 5′-GGTTTGATCTTGGTAGGG-3′ were used. To amplify GAPDH gene, the forward primer as 5′-AGGTCGGTGTGAACGGATTTG-3′ and the reverse primer as 5′-TGTAGACCATGTAGTTGAGGTCA-3′ were used. Fluorescence detection was conducted at the extension stage. Each reaction was performed in quadruplicate, and TH gene expression was normalized to that of GAPDH.
Flow cytometry analysis and ELISA
Flow cytometry analysis was performed using the FACSCalibur cytometer (BD Bioscience, San Jose, CA) in splenocytes. Monoclonal antibody for mouse F4/80 (PE, BM8) and CD11b (APC, M1/70) was used, and Flowjo v10 (Flowjo LLC, Ashland, OR, USA) was used for analysis.
ELISA test was conducted using the corticosterone ELISA kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions.
Western blotting
Ten micrograms of total protein from mammary gland and Stat3 (79D7) and pStat3 (Ser727) monoclonal antibodies was used as primary antibodies. After incubation with HRP-conjugated secondary antibody, chemiluminescence signal detection was conducted. Antibodies for Western blotting were purchased from Cell Signaling Technology (Boston, MA).
Statistical analysis and criteria for premature mammary gland involution
Statistical analysis was conducted with GraphPad Prism version 5.02 (GraphPad Software Inc., La Jolla, CA, USA) for nonparametric t-tests. In addition, simultaneous mammary gland epithelial cell apoptosis and nursed pup alopecia in dam was designated as premature mammary gland involution.
Results
Repeated corticosterone injection induced apoptosis and fat deposition in adipocyte in mammary gland of B6.IL-10
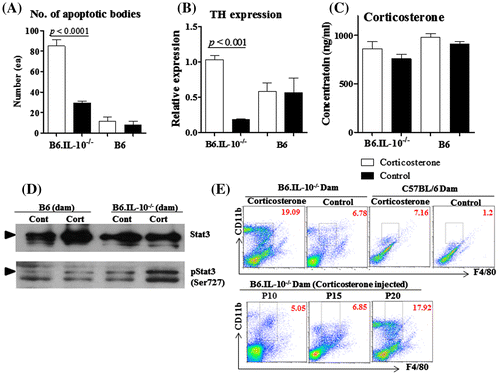
In histological examination, B6.IL-10−/− dams with corticosterone exhibited many apoptotic bodies in their lumen of mammary gland. Chromatin-condensed bodies were observed in H&E, and they were TUNLE positive, but there were few keratin 18-positive cells in mammary gland. This indicated that apoptosis happened in keratin 18 mammary gland epithelial cells (Fig. ). Control B6.IL-10−/− and both group of B6 also showed apoptotic bodies in their lumen of mammary gland, but relatively small numbers than that of B6.IL-10−/− with corticosterone. In detail, there was almost threefold number of apoptotic bodies in the lumen of B6.IL-10−/− with corticosterone as 85.2 ± 15.1 than control B6.IL-10−/− as 29.4 ± 47 (p < 0.001), and no significant difference was observed between B6 dams as 11.3 ± 10.3 in corticosterone-treated mice and 8.3 ± 8.0 in control mice. In comparison between control group, B6.IL-10−/− showed about threefold number of apoptotic bodies than B6 (Fig. (A)).
Fig. 1. Histological examination for mammary glands in P19.
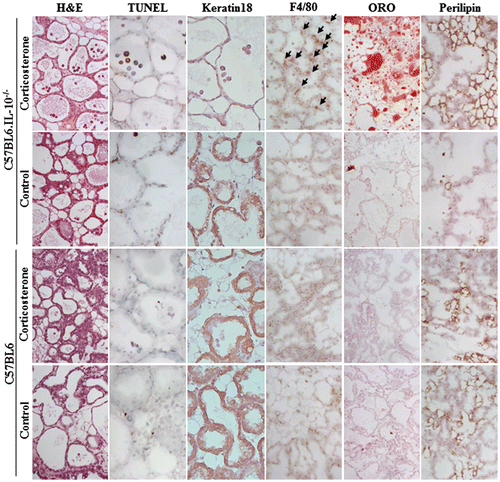
Fig. 2. Mammary gland involution in corticosterone-injected B6.IL-10−/− mice.
The second definitive change was fat deposition in adipocyte in mammary gland. In ORO staining, the B6.IL-10−/− with corticosterone exhibited remarkable fat deposition in adipocyte, but other groups did not. In consistent with ORO staining, B6.IL-10−/− with corticosterone showed numerous perilipin-positive adipocyte, and control B6.IL-10−/− showed few adipocytes. In B6 dams, there were no ORO-positive adipocytes, but a few perilipin-positive adipocytes (Fig. ).
Corticosterone injection induced premature mammary gland involution under interleukin 10 deficiency
Quantitative RT-PCR for TH gene expression in adrenal gland was conducted to assess the stress response of corticosterone injection. Corticosterone injection resulted in TH gene in adrenal gland up-regulation in B6.IL-10−/−, but not in B6. In comparison between B6.IL-10−/− mice, corticosterone-injected dam showed about fivefold increase in TH gene expression than control dam in adrenal gland as 1.03 ± 0.05 vs. 0.18 ± 0.06 (p < 0.001). There was similar tendency in TH gene in adrenal gland expression and number of apoptotic bodies that B6.IL-10−/− with corticosterone showed significantly high than control dams, but B6 did not show significance (Fig. (B)). Although serum corticosterone concentration was relatively high in corticosterone mice than control group, it was not significantly different. Serum corticosterone concentrations were 860.5 ± 77.5 and 758.3 ± 47.5 ng/ml in B6.IL-10−/− with corticosterone and control mice, and 979.3 ± 38.0 and 908.0 ± 26.7 ng/ml in B6 with corticosterone and control mice. B6.IL-10−/− with corticosterone did not show significance high than other dams (Fig. (C)).
Mammary gland alveolar cell apoptosis is a hallmark of mammary gland involution. To confirm apoptosis, Western blotting for Stat3 and Stat3 phosphorylation was conducted, and high Stat3 phosphorylation was observed in B6.IL-10−/− with corticosterone (Fig. (D)).
Increasing of macrophage cells in premature mammary gland involution
In immune stain for mammary gland tissue, macrophage (F4/80) infiltration was observed in B6.IL-10−/− dams with corticosterone. Consistent with apoptotic change, control B6.IL-10−/− and both groups of B6 dams did not show typical immune cell infiltration (Fig. ).
In flow cytometry analysis using the splenocyte, B6.IL-10−/− dam with corticosterone showed about 3 times macrophage populations (F4/80 and CD11b double-positive cells) than B6.IL-10−/− control. B6 dam with corticosterone also showed similar increasing pattern of macrophage population. This might indicate that corticosterone injection increases macrophage population in B6.IL-10−/− and B6, and overall macrophage population was 2 times higher in B6.IL-10−/− dams than in B6 dams. In addition to analysis in 3 different postnatal days (P10, P15, and P20), macrophage population gradually increased, and there seemed to be sharp increase during P15–P20 (Fig. (E)).
Premature mammary gland involution caused pup alopecia
Pup alopecia started at 15–16 days old (d), and severity and onset of alopecia was similar among littermates. At the onset of alopecia, diffuse hair loss on the dorsal trunk was observed, and hair loss developed throughout the body except for the head (Fig. (A)). Overall incidence of pup alopecia was relatively high in 57% (4 of 7 dams) in B6.IL-10−/− with corticosterone than 20% (1 of 5 dams) control B6.IL-10−/−. There was no pup alopecia in B6 dams with corticosterone and control group (Fig. (B)). All dams with alopecia pups showed mammary gland epithelial cell apoptosis. These results indicated that premature mammary gland involution developed pup alopecia in B6.IL-10−/−. In H&E stain for pup skin, there were follicular cyst formation, mild hyperkeratosis, and broken hair. This was similar to symptoms observed in previous studies.Citation10,16,17) In addition, significant macrophage (F4/80-positive cells) infiltration was observed in the epidermis region of alopecia pups. Control B6.IL-10−/− and both group of B6 appeared normal (Fig. (C)).
Fig. 3. Corticosterone-injected dam developed pup alopecia in B6.IL-10−/−.
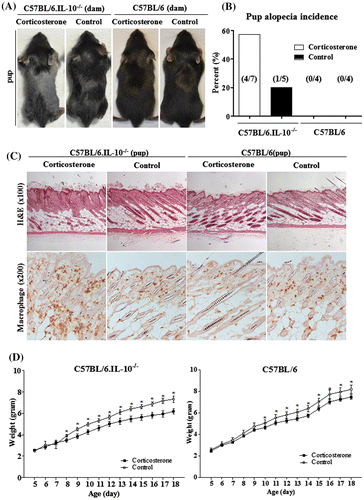
Corticosterone injection developed approximately 14% growth retardation in B6.IL-10−/− and about 9% in B6. Growth retardation started from P7 to P10 in B6.IL-10−/− and B6 (Fig. (D)).
Discussion
This study demonstrated that only interleukin 10-deficient mice developed premature mammary gland involution. Similar to previous report, IL-10 plays an important role in preventing depression by regulating corticosterone production by the hypothalamic–pituitary–adrenal axis;Citation18) thus, IL-10-deficient mice may be more susceptible to stress. TH gene is rate-limiting enzyme in the dopamine and noradrenergic system, and gene expression increase in stress situation.Citation19) Quantitative PCR for TH gene mRNA showed corticosterone injection-induced upregulation of TH mRNA in adrenal gland only in B6.IL-10−/− dams. B6 wild-type mice with corticosterone did not show upregulation of the TH gene in adrenal gland, and this result agrees with the hypothesis that IL-10 is important for stress regulation; thus, only IL-10-deficient dams under excessive stress develop premature mammary gland involution.
Premature mammary gland involution in B6.IL-10−/− dams was confirmed by mammary gland epithelial cell apoptosis, fat deposition in adipocyte, macrophage infiltration, and significant phosphorylation of Stat3.Citation20) ORO stain was used for detecting neutral triglyceride, and perilipin is a protein that coat lipid droplet.Citation21) In B6.IL-10−/−, ORO and perilipin stain showed similar results that there were many fat deposition in corticosterone group. However, B6 dams showed several perilipin-positive adipocytes but no ORO-positive fat droplet. The cause of this difference was uncertain, but authors assumed that B6 mice were not under mammary gland involution in P19, so lipid droplet did not enlarge and was too small for ORO staining.
Briefly, impaired or deficient IL-10 induced failure of stress regulation and finally caused mammary gland epithelial cell apoptosis. Thus, B6.IL-10−/− seemed to be a good model for stress regulation. In addition, it was demonstrated that simultaneous mammary gland epithelial cell apoptosis and pup alopecia could be criteria of maternal premature mammary gland involution in corticosterone-injected B6.IL-10−/−. About 60% of B6.IL-10−/− with corticosterone and 20% of control B6.IL-10−/− developed premature mammary gland involution. We assumed that simple injection could be stressful to IL-10-deficient mice, and IL-10 might affect gene regulation relating Stat3 phosphorylation. However, role of IL-10 for mammary gland apoptosis by regulating important genes, such as Lif, TGFb3, and IL-6, was not obvious.
Even though corticosterone injection seemed to increase macrophage infiltration in B6 and B6.IL-10−/−, B6.Il-10−/− with premature mammary gland involution showed remarkable increase in macrophage infiltration in mammary gland and almost threefold high frequency in spleen. Increase in the macrophage population during mammary gland involution was consistent with previous report.Citation22) Macrophage is important for epithelial cell death, clearance of apoptotic bodies, and adipocyte repopulation.Citation8,22) In this study, macrophage population rapidly increase from P15 to P20 in dams, and pup alopecia started at 15–16 days, and all B6.IL-10−/− dams with alopecia pup, showed premature mammary gland involution. This result might indicate that premature mammary gland involution started around P15 in B6.IL-10−/− with repeated corticosterone injection. Initiation of mammary gland involution is milk stasis stress in normal condition, but premature mammary gland involution B6.IL-10−/− with corticosterone still nursed their pups; in other words, there was less or no milk stasis. This result showed that mammary gland involution could happen not only by milk stasis but also by excessive stress or cytokine deficient. As similar to previous studies, apoptotic bodies from mammary gland were administered via maternal milk and cause skin inflammation and alopecia in pups.Citation10)
In conclusion, we demonstrated that IL-10 is important for stress modulation, and impaired IL-10 function can cause premature mammary gland involution in a mouse model with external stress. This premature mammary gland involution model in B6.IL-10−/− with corticosterone has several advantage such as simple to establish, well-defined onset of mammary gland involution, high incidence, and inducing pup alopecia.
Author contributions
Woo-Sung Hwang contributed to conducting experiment and writing the manuscript. Ji-Hyun Bae contributed to conducting experiment. Su-Cheong Yeom contributed to designing the experiment and discussion of the data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Basic Science Research Program by the National Research Foundation of Korea [grant number 2012R1A1A2042581].
References
- Green KA, Streuli CH. Apoptosis regulation in the mammary gland. Cell Mol. Life Sci. 2004;61:1867–1883.10.1007/s00018-004-3366-y
- Watson CJ. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 2006;8:1–5.10.1186/bcr1401
- Flanders KC, Wakefield LM. Transforming growth factor-βs and mammary gland involution; functional roles and implications for cancer progression. J. Mammary Gland Biol. Neoplasia. 2009;14:131–144.10.1007/s10911-009-9122-z
- Tiffen PG, Omidvar N, Marquez-Almuina N, et al. A dual role for oncostatin M signaling in the differentiation and death of mammary epithelial cells in vivo. Mol. Endocrinol. 2008;22:2677–2688.10.1210/me.2008-0097
- Kritikou EA, Sharkey A, Abell K, et al. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development. 2003;130:3459–3468.10.1242/dev.00578
- Zhao L, Melenhorst JJ, Hennighausen L. Loss of interleukin 6 results in delayed mammary gland involution: a possible role for mitogen-activated protein kinase and not signal transducer and activator of transcription 3. Mol. Endocrinol. 2002;16:2902–2912.10.1210/me.2001-0330
- Kreuzaler PA, Staniszewska AD, Li W, et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat. Cell Biol. 2011;13:303–309.10.1038/ncb2171
- Stein T, Morris JS, Davies CR, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–91.10.1186/bcr753
- Sohn BH, Moon HB, Kim TY, et al. Interleukin-10 up-regulates tumour-necrosis-factor-α-related apoptosis-inducing ligand (TRAIL) gene expression in mammary epithelial cells at the involution stage. Biochem. J. 2001;360:31–38.10.1042/bj3600031
- Hwang WS, Kim HI, Kim YJ, et al. Pregnancy in postpartum estrus induces inflammatory milk production and catagen specific pup skin inflammation in interleukin-10 deficient mice. J. Dermatol. Sci. 2013;72:225–232.10.1016/j.jdermsci.2013.07.004
- Green KA, Nielsen BS, Castellino FJ, et al. Lack of plasminogen leads to milk stasis and premature mammary gland involution during lactation. Dev. Biol. 2006;299:164–175.10.1016/j.ydbio.2006.07.021
- Yoo KH, Oh S, Kang K, et al. Loss of EZH2 results in precocious mammary gland development and activation of STAT5-dependent genes. Nucleic Acids Res. 2015;43:8774–8789.10.1093/nar/gkv776
- Hanayama R, Nagata S. Impaired involution of mammary glands in the absence of milk fat globule EGF factor 8. roc. Natl. Acad. Sci. U.S.A. 2005;102:16886–16891.10.1073/pnas.0508599102
- Zhao Y, Ma R, Shen J, et al. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008;581:113–120.10.1016/j.ejphar.2007.12.005
- Kinkel AD, Fernyhough ME, Helterline DL, et al. Oil red-O stains non-adipogenic cells: a precautionary note. Cytotechnology. 2004;46:49–56.10.1007/s10616-004-3903-4
- Vanderford DA, Greer PK, Sharp JM, et al. Alopecia in IL-10-deficient mouse pups is c-kit-dependent and can be triggered by iron deficiency. Exp. Dermatol. 2010;19:518–526.10.1111/exd.2010.19.issue-6
- Wan Y, Saghatelian A, Chong LW, et al. Maternal PPAR protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007;21:1895–1908.10.1101/gad.1567207
- Roque S, Correia-Neves M, Mesquita AR, et al. Interleukin-10: a key cytokine in depression? Cardiovasc. Psychiatry Neurol. 2009;2009:187894.
- Kiss A, Mravec B, Palkovits M, et al. Stress-induced changes in tyrosine hydroxylase gene expression in rat hypothalamic paraventricular, periventricular, and dorsomedial nuclei. Ann. N.Y. Acad. Sci. 2008;1148:74–85.10.1196/nyas.2008.1148.issue-1
- Chapman RS, Lourenco PC, Tonner E, et al. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 1999;13:2604–2616.10.1101/gad.13.19.2604
- Kern PA, Di Gregorio G, Lu T, et al. Perilipin expression in human adipose tissue is elevated with obesity. J. Clin. Endocrinol. Metab. 2004;89:1352–1358.10.1210/jc.2003-031388
- O’Brien J, Martinson H, Durand-Rougely C, et al. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development. 2012;139:269–275.10.1242/dev.071696
