Abstract
Salivary IgA—a primary factor in local immunity of the oral cavity—plays an important role in maintaining local immune function in the oral cavity and prevent upper respiratory tract infections. Oral IgA levels are known to fluctuate in an exercise-dependent manner; thus, we investigated the effects of voluntary exercise on salivary IgA secretion in rats to better understand the mechanism by which this occurs. Six-week-old male Wistar rats were placed in individual cages with or without access to exercise wheels for three weeks. Notably, animals who engaged in voluntary exercise demonstrated significant increases in IgA concentration in saliva and submandibular gland tissue, as well as a markedly higher salivary IgA flow rate. Moreover, active rats also exhibited elevated polymeric Ig receptor (pIgR) mRNA expression in submandibular gland tissue. Collectively, these results suggest that voluntary exercise may increase salivary IgA concentration and boost immune function in the oral cavity.
Graphical Abstract
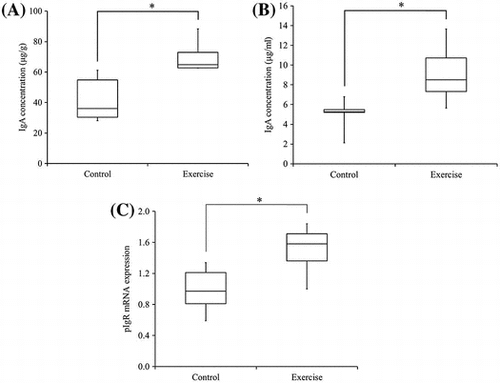
Voluntary exercise of rats increased IgA concentration in the submandibular gland (A) and saliva (B). It also demonstrated higher pIgR mRNA expression in submandibular gland (C).
The oral cavity is constantly in contact with the outside world; as such, the oral mucosa serves as the body’s first line of immunological defense. Saliva secreted by the salivary glands plays the most important role in this defense mechanism and includes a large amount of IgA.Citation1−3) Salivary IgA is known to fluctuate in response to changes in immune function, as well as with exercise,Citation4,5) and is reported to be greatly involved in the prevention upper respiratory tract infections.Citation6−8)
Nearly all IgA in human serum is monomers composed of two heavy chains (α chains) and two light chains (κ chains and λ chains). In contrast, approximately 90% of the IgA in saliva exists in a polymeric form (dimers or trimers) composed of monomers linked by a joining chain (J chain) Citation2); thus, nearly all salivary IgA is secreted by the salivary glands rather than being derived from serum. During this process, polymeric IgA is synthesized and secreted by immunoglobulin-producing plasma cells in the tissue surrounding the salivary glands, binds with polymeric Ig receptor (pIgR) expressed in the basal surface of salivary gland acinar cells, and is then transported via intracellular transcytosis to the luminal surface. Here, a portion of the pIgR is then uncoupled to become the secretory component, which can then be released into the saliva as secretory IgACitation2,9−11) and form immune complexes with invading viruses, bacteria, bacterial toxins, and allergens to eliminate them from the body.Citation9−13)
All three major human salivary glands (the submandibular, sublingual, and parotid) secrete IgA into saliva and contain immunoglobulin-producing plasma cells and pIgR-expressing acinar cells.Citation14,15) The daily amount of saliva secreted is 1000–1500 mL; at rest, approximately 70% of saliva is secreted from the submandibular glands, while 25% is secreted from the parotid glands, and 5% is secreted from the sublingual glands, and collectively contains 50–200 mg IgA.Citation2,16)
Many studies have reported the effect of exercise on the risk of upper respiratory tract infection.Citation6−8,17,18) The relationship between exercise and upper respiratory tract infection sensitivity can be demonstrated with a J curve model,Citation6) which shows that sedentary individuals can reduce their risk of infection by engaging in exercise until a threshold at which point excessive exercise becomes detrimental to health. Notably, excessive exercise results in a temporary reduction in salivary IgA concentration, as well as in the abundance and function of peripheral NK and T cells. Concurrently, immunosuppressive stress hormones and inflammatory cytokines are secreted that can increase susceptibility to infection.Citation6,19−21)
Nearly all studies on exercise and salivary IgA have been conducted with human subjects. Many of these studies have reported that moderate exercise increases salivary IgA levels, whereas intense exercise reduces salivary IgA levels and weakens mucosal immunity.Citation18,22−24) In an experiment using an animal model and a treadmill, Kimura et al. found that intense forced exercise significantly reduced salivary IgA concentrations and glandular pIgR mRNA expression, and inhibited IgA secretion into saliva, thereby reducing salivary IgA concentration.Citation25)
In animal experiments on exercise and immunity, a number of methods have been used to make laboratory animals exercise. These methods include: forced running on a treadmill,Citation25−28) forced swimming in a tank,Citation29) semi-forced climbing in a wire mesh cage,Citation30,31) and encouragement of voluntary running on an exercise wheel.Citation32,33) Aside from voluntary running on an exercise wheel, all of these methods impose forced exercise that may provoke a strong physiological stress response and thus greatly affect secretion of saliva. Moraska et al. reported that while treadmill running has positive physiological effects, such as reducing body weight and increasing muscle energy metabolism efficiency, it also yields negative effects such as adrenal hypertrophy, thymic involution, and immunosuppression.Citation28) The advantages of voluntary running on an exercise wheel as given by Holy et al. include the absence of unnecessary stress; the minimal change in the environment between exercising and not exercising; and allowing the animals to exercise naturally at times when they would normally engage in brisk physical activity.Citation33) Thus, voluntary exercise is considered to have the same significance in daily life for rats as for humans.
In the present study, we sought to determine the effect of non-forced, voluntary exercise (i.e. exercise which does not impose unnecessary stress) on salivary IgA secretion in laboratory animals which has not yet been addressed.
Materials and methods
Animals
Twenty 6-week-old male Wistar rats (Japan CLEA, Tokyo, Japan) were housed in individual cages each equipped with an exercise wheel (Shinano Seisakusho, Tokyo, Japan; 370 mm diameter, 100 mm width) at 22 ± 3 °C under a 12-h light/12-h dark cycles. Indigestible carbohydrates have been reported to increase IgA in the submandibular glands and saliva of rats;Citation34) therefore, a special fiber-free diet (see next section on “Diet”) and water were provided ad libitum to eliminate any dietary effects. The rats were housed in cages for one week with the door leading to the exercise wheel closed to obtain baseline IgA samples from the submandibular glands and saliva. Then, the rats were divided into two groups (exercise group: n = 5 × 2, control group: n = 5 × 2). For the exercise group, the door leading to the exercise wheel was left open and the rats were allowed to exercise voluntarily for the three weeks until the end of the experiment, whereas the doors remained closed in control cages. All study protocols were approved by the Ethics Committee for Animal Experiments of Kanagawa Dental University and conducted in strict adherence to the Guidelines for Animal Experimentation of Kanagawa Dental University and ARRIVE guidelines for reporting animal research.
Diet
The composition of the special fiber-free diet is shown in Table . The diet was based on the solidified AIN-76 formulation, with all maize starch and cellulose replaced by sucrose. This diet was made by Japan CLEA.
Table 1. The composition (%) of the fiber-free diet and the AIN-76 diet that was the basis of this diet.
Collection of specimens
Following the conclusion of the exercise period, the rats received intraperitoneal injections of pentobarbital (65 mg/kg body weight; Kyoritsu Seiyaku Corporation, Tokyo, Japan), and the samples were then collected.
Collection of submandibular glands and serum
Thoracotomies were performed on anesthetized rats, and blood was collected via cardiocentesis. Collected blood was quickly injected into evacuated blood collection tubes containing serum separation medium (Venoject II; Terumo Corporation, Tokyo, Japan), mixed by inversion 5–6 times, and then stored in ice. The tubes were subsequently centrifuged (1200 × g, 20 min, 4 °C) to obtain serum, which was then quickly frozen in liquid nitrogen. Following blood collection, the rats were decapitated and the bilateral submandibular glands were collected and quickly frozen in liquid nitrogen. All specimens were stored at −80 °C until analysis.
Collection of saliva
Anesthetized rats were given intraperitoneal injections of pilocarpine (1.5 mg/kg; Nacalai Tesque, Inc., Kyoto, Japan) to induce salivation. Five min later, secreted saliva in the oral cavity was collected over a 10-min period with a micropipette, quickly frozen in liquid nitrogen, and stored at −80 °C until analysis. Salivary IgA flow rate was determined by dividing the weight of saliva by the sampling time, assuming that the specific gravity of saliva was 1.00.
Measurement of IgA concentrations
Concentrations of IgA present in submandibular glands, serum, and saliva were measured with a Rat IgA ELISA Quantitation Kit (Bethyl Laboratories, Montgomery, Texas, USA). The full mass of the right side of the submandibular glands was used. A cryo-press (Microtec Company Limited, Chiba, Japan) was used to crush the frozen specimens, which were then mixed with phosphate-buffered saline (0.01 M, pH 7.2–7.4, Wako Pure Chemical Industries, Osaka, Japan) containing 1 mM phenylmethylsulfonyl fluoride dissolved in 1% Triton® X-100 (MP Biomedicals, LLC, Santa Ana, USA) and dimethyl sulfoxide (DMSO). This solution was centrifuged (10,000 × g, 15 min, 4 °C) and the resulting supernatant was used in ELISA assays.
For ELISA, goat anti-rat IgA antibody (primary antibody) diluted 100 times with a solution containing 0.05 M carbonate-bicarbonate, pH 9.6 and added to every well of a 96-well microplate, which was left to sit at room temperature for 1 h. The remaining primary antibody solution was then removed and plates were washed five times with a washing solution (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, pH 8.0) prior to blocking in 50 mM Tris, 0.14 M NaCl, and 1% bovine serum albumin, pH 8.0. After sitting at room temperature for 30 min, the microplate was washed five times with the above-described washing solution. The diluted specimen solution and rat IgA standard solution (Bethyl Laboratories, Montgomery, Texas, USA) were added to each well, incubated at room temperature for 1 h, and then washed five times with washing solution. Horseradish peroxidase-conjugated goat anti-rat IgA antibody was diluted 50,000 times and added to each well. After a 1-h incubation at room temperature, plates were washed five times with washing solution and a colorimetric substrate containing TMB was added to each well. Reactions were left in the dark at room temperature for 15 min, at which point a quenching solution (0.18 M H2SO4) was added to each well to stop the reactions. Absorbance was measured with a microplate reader (Bio-Rad, Hercules, California, USA) at a wavelength of 450 nm. Concentrations of IgA were determined by creating a calibration curve (standard curve) and calculating concentrations based on this calibration curve and the absorbance of the samples.
RNA extraction and cDNA synthesis
RNA was extracted from one-quarter of the left submandibular gland using ISOGEN reagent (Nippon Gene Corporation, Toyama, Japan) per the manufacturer’s protocol. RNA concentrations were measured using a BioSpec-nano spectrophotometer (Shimadzu Access Corporation, Kanagawa, Japan). Complementary DNA (cDNA) was synthesized from total RNA using a first-strand cDNA synthesis kit (Roche Diagnostics Ltd, Lewes, UK).
Measurement of submandibular gland pIgR mRNA by quantitative real-time PCR
Real-time PCR was performed using a LightCycler 480 system (Roche Applied Science, Mannheim, Germany) per the manufacturer’s protocol. Reactions were performed in 20 ea volumes (0.5 μmol of each primer, 0.1 μmole TaqMan probe). The sequence of primers for amplifying the pIgR gene sequence were as follows: forward primer: 5′-TGG GAG CTA CAA GTG TGG TC-3′, reverse primer: 5′-GGG TGT CAT TTG GGA ATC CAG-3′. The TaqMan probe sequence was FAM-5′ TTC GAT GTC AGC CTG GAG GTC AGC-3′-TAMRA, and was designed and synthesized at Nippon Gene Research Laboratory. To amplify pIgR, PCR was performed at 95 °C for 10 min, followed by 45 cycles of 95 °C for 10 s and 62 °C for 30 s. qRT-PCR for rat β-actin gene expression was performed using FastStart DNA Master SYBR Green I (Roche Diagnostics. Ltd) and a LightCycler 480 system per the manufacturer’s protocol. The rat β-actin primer sequences were as follows: forward primer: 5′-CTT GTA TGC CTC TGG TCG TA-3′, reverse primer: 5′-CCA TCT CTT GCT CGA AGT CT-3′. Denaturation was performed at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 10 s. Gene expression was determined by the ratio of pIgR mRNA expression and β-actin mRNA expression in each sample.
Statistical analysis
The mean body weight and weight gain of the rats were analyzed with Student’s t-test. All others were analyzed with Mann–Whitney U tests. Statistical analysis was performed in SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) and significance was defined as p < 0.05.
Results
Rat body weight
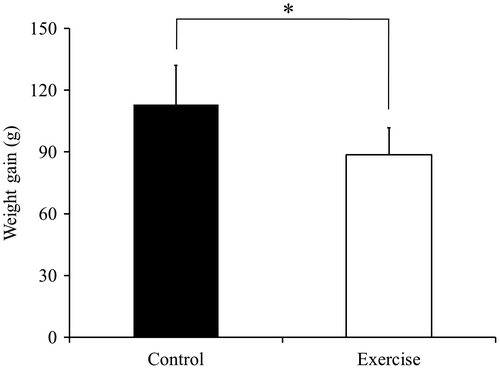
The mean body weight at the start of the experiment in the control group and in the exercise group was 208.8 ± 7.3 g and 198.8 ± 7.9 g, and these values were no significantly different. A comparison between the exercise group and the control group revealed a significant difference in weight gain (p < 0.05; Fig. ). All values show the mean ± standard deviation (SD).
IgA concentrations in rat submandibular gland tissue
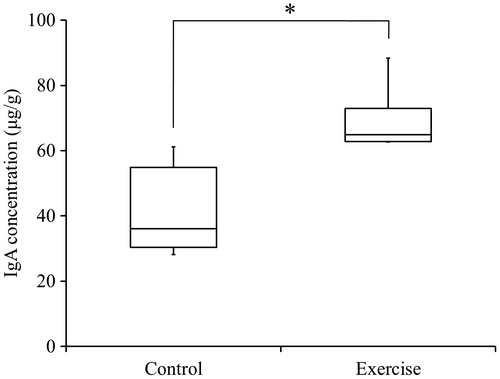
The exercise group demonstrated significantly higher IgA concentrations in submandibular gland tissue when compared to the control group (p < 0.05; Fig. ). Hypertrophy of the tissue in response to voluntary exercise was not observed.
Fig. 2. Concentrations of IgA in rat submandibular gland tissue.
IgA concentrations in rat serum
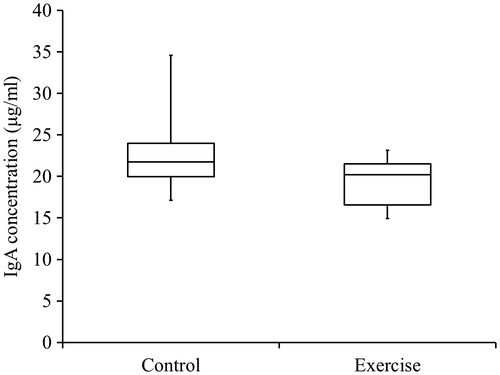
No significant differences were observed in serum IgA concentrations between the exercise and control groups (Fig. ).
IgA concentrations in rat saliva
The exercise group demonstrated significantly higher salivary IgA concentrations compared to controls (p < 0.05; Fig. ).
Fig. 4. Concentrations of IgA in rat saliva.
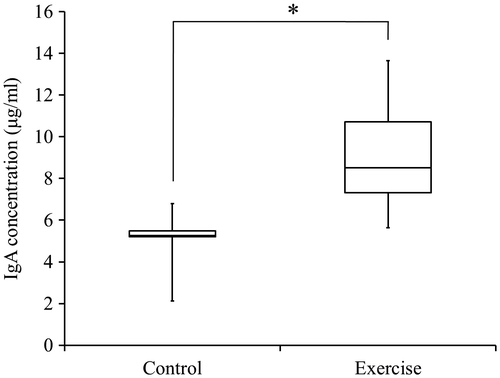
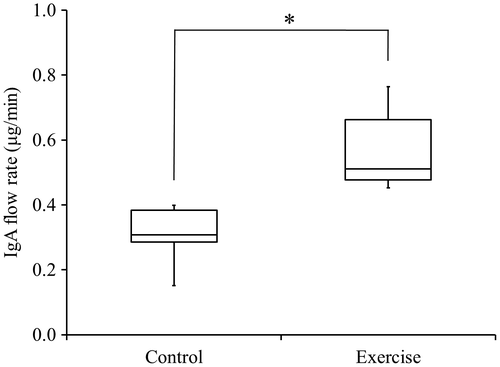
Salivary flow rate and Salivary IgA flow rate
The salivary flow rate of the rats, as measured for 10 min, did not show significant differences between the control group and the exercise group. The exercise group demonstrated a significantly faster flow rate of salivary IgA than did the control group (p < 0.05; Fig. ).
Fig. 5. Flow rate of IgA in rat saliva.
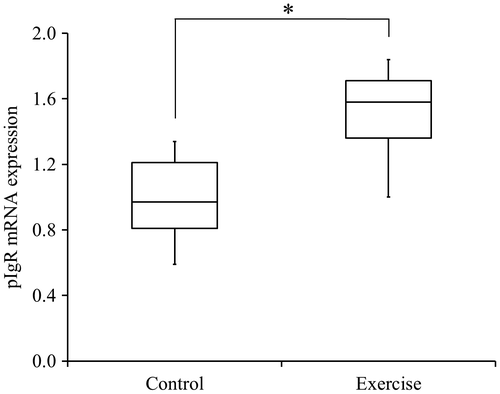
pIgR mRNA expression in rat submandibular gland tissue
The exercise group demonstrated significantly greater pIgR mRNA expression (pIgR/β-actin) in submandibular gland tissue than did the control group (p < 0.05; Fig. ).
Fig. 6. Expression of pIgR mRNA in rat submandibular gland tissue (pIgR/β-actin)
Discussion
The relationship between exercise and immunity is important in areas such as infection prevention. In addition, as society continues to age, exercise is garnering greater attention as a means of preventing age-related decline in immune function. There have been many studies on exercise and immunity, most of which have involved human subjects.Citation6−8,18,19) An appropriate level of exercise is considered to boost immune function and be effective in preventing infections and cancer, whereas excessive exercise is considered to reduce immune function and promote inflammation and allergies.Citation35−38)
In the present study, the salivary IgA concentration increased when rats that were fed a fiber-free diet engaged in voluntary exercise. In a previous study by Yamamoto et al., similar to the results of the present study, the salivary IgA concentration of rats fed a fructooligosaccharide-containing fiber-free diet was higher than that of rats fed a simple fiber-free diet.Citation34) Fructooligosaccharide possibly changes the composition of the intestinal flora, and the findings of our previous study suggest that indigestible carbohydrates in the diet affects salivary IgA concentration. Citation34) The increase in salivary IgA concentration by voluntary exercise observed in this study may be a phenomenon that occurs only under conditions where the decrease in salivary IgA concentration occurs because of deficiency of indigestible carbohydrates. In other words, voluntary exercise may have positively affected on the salivary IgA concentration instead of indigestible carbohydrate intake, possibly through changes in intestinal flora in a similar way that is caused by indigestible carbohydrate intake. In fact, the composition of the intestinal flora is reported to vary with voluntary exercise.Citation32,39) However, in order to clarify these hypotheses, the following experiments will be required: (1) to test the effects of voluntary exercise on salivary IgA concentration in rats fed a diet including indigestible carbohydrates, (2) to compare the composition of microflora in rats under two conditions (indigestible carbohydrate intake vs. voluntary exercise), (3) to test the effect of voluntary exercise on salivary IgA in germ-free rats.
The exercise group and the control group exhibited significant differences in body weight change from the beginning to the conclusion of the exercise period. Because the dietary intake is considered to have been increased by exercise,Citation40) this result obtained from voluntary exercise on an exercise wheel demonstrates that voluntary exercise occurred in the exercise group. We also found that exercise did not yield an increase in IgA concentration in serum. The systemic immune system and the mucosal immune system, which differ in terms of the distribution and secretion of antibody isotypes, both possess independent IgA production mechanisms.Citation2) Considering that the same has also been reported in humansCitation41) and the limited transfer of IgA from serum to saliva, the absence of a relationship between salivary IgA and serum IgA indicates that increases in IgA are local to the submandibular glands.
Exercise is a type of physiological stress. Exercise-induced changes in immune function are regulated not by the immune system alone, but by its interactions with the nervous system and the endocrine system.Citation42,43) Stress information is relayed from the cerebral cortex-limbic system to the hypothalamus, and then from the hypothalamus to the entire body via the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis (HPA axis). In the sympathetic nervous system, sympathetic nerve stress results in the secretion of catecholamines (adrenaline and noradrenaline) from the adrenal medulla increases, thereby yielding various effects on the entire body.Citation42) Various types of hormones have also been determined to act on immune cells in the HPA axis.Citation43) Thus, the main difference between forced exercise and voluntary exercise is the difference in stress response. Forced exercise imposes severe stress, thus resulting in the secretion of large amounts of stress hormones. These stress hormones in turn inhibit the abundance and function of immune cells, and ultimately diminish immune function. Hindered immune function was not observed in the voluntary exercise model used in the present study, which may have been strongly affected by the immune function-boosting effect of exercise itself.
Many studies have reported that exercise induces the release of various cytokines from immune cells throughout the body.Citation44−47) During the induction of IgA-producing cells, IL-5 and IL-6 (secreted by Th2 cells, which are related to humoral immunityCitation48,49)) are involved in the differentiation of B cell precursors of IgA.Citation50) Helper T cell-producing cytokines are closely involved with IgA induction, which plays the main role in mucosal immunity. The results of the present study indicate that this mechanism may have been affected by voluntary exercise.
pIgR is a special transport protein that plays an important role in acinar cell-mediated intracellular transport of IgA in submandibular gland tissue.Citation2,9−11) Reduced pIgR expression in the submandibular gland tissue suppresses the secretion of IgA into saliva, thereby reducing salivary IgA concentration.Citation25) Therefore, increased pIgR expression in submandibular gland tissue is considered to increase salivary IgA concentration. Consequently, the voluntary exercise-induced increase in salivary IgA concentration in the present experiment is surmised to have been the result of increased pIgR expression. pIgR expression is affected by a variety of factors throughout the body. Locally, cytokines produced by mucosal tissue and exocrine glands increase pIgR expression additively and synergistically.Citation13) Of these cytokines, INF-γ and IL-4 have been reported to synergistically increase pIgR.Citation51) Exercise has frequently been reported to affect these cytokines, as well as the helper T cells which produce them.Citation46,47) It is surmised that in the present study, voluntary exercise, which involves little stress, may have yielded a synergistic effect of INF-γ and IL-4, which normally act antagonistically.
In conclusion, we determined that voluntary exercise induces a local increase in IgA concentration in the submandibular glands, and that this IgA is secreted into saliva as secretory IgA. We also determined that voluntary exercise exerts a major effect on pIgR expression in submandibular gland tissue, resulting in an increase in salivary IgA concentration. These findings suggest that voluntary exercise may boost immune function in the oral cavity.
Author contributions
The authors’ contributions were as follows: Yuki Kurimoto conceived, designed and performed the experiments, analyzed the data, and wrote the article. Juri Saruta performed the experiments and wrote the article. Masahiro To performed the experiments. Yuko Yamamoto performed the experiments. Koji Kimura performed the experiments. Keiichi Tsukinoki designed the experiments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported, in part, by a Kakenhi Grant-in-Aid for Young Science (B) [#26861582] to J.S. and a Challenging Exploratory Research grant [#26670821] to K.T. from the Japan Society for the Promotion of Science.
Notes
Abbreviations: pIgR, polymeric Ig receptor; ELISA, enzyme-linked immunosorbent assay; PCR, Polymerase chain reaction; NK, natural killer; DMSO, dimethyl sulfoxide; PMSF, phenylmethylsulfonyl fluoride; PBS, phosphate buffered saline; HRP, horseradish peroxidase; TMB, tetramethylbenzidine; HPA, hypothalamic- pituitary-adrenal axis; Th, T helper; IL, interleukin; INF, interferon.
References
- Lamm ME, Nedrud JG, Kaetzel CS, et al. IgA and mucosal defense. APMIS. 1995;103:241–246.10.1111/apm.1995.103.issue-1-6
- Mazanec MB, Nedrud JG, Kaetzel CS, et al. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today. 1993;14:430–435.10.1016/0167-5699(93)90245-G
- Kotani Y, Shinkai S, Okamatsu H, et al. Oral intake of Lactobacillus pentosus strain b240 accelerates salivary immunoglobulin a secretion in the elderly: a randomized, placebo-controlled, double-blind trial. Immun. Ageing. 2010;7:11.10.1186/1742-4933-7-11
- Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc. Immunol. Rev. 2011;17:6–63.
- Otsuki T, Shimizu K, Iemitsu M, et al. Chlorella intake attenuates reduced salivary SIgA secretion in kendo training camp participants. Nutr. J. 2012;11:103.10.1186/1475-2891-11-103
- Nieman DC. Exercise, upper respiratory tract infection, and the immune system. Med. Sci. Sports. Exerc. 1994;26:128–139.10.1249/00005768-199402000-00002
- Nieman DC, Nehlsen-Cannarella SL, Markoff PA, et al. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int. J. Sports. Med. 1990;11:467–473.10.1055/s-2007-1024839
- Nieman DC. Exercise, infection, and immunity. Int. J. Sports. Med. 1994;15:S131–S141.10.1055/s-2007-1021128
- Asano M, Komiyama K. Polymeric immunoglobulin receptor. J. Oral. Sci. 2011;53:147–156.10.2334/josnusd.53.147
- Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484.10.1016/j.vaccine.2006.12.001
- Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand. J. Immunol. 2009;70:505–515.10.1111/sji.2009.70.issue-6
- Corthésy B. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Future Microbiol. 2010;5:817–829.10.2217/fmb.10.39
- Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005;206:83–99.10.1111/imr.2005.206.issue-1
- Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann. N. Y. Acad. Sci. 2007;1098:288–311.10.1196/annals.1384.012
- Carpenter GH, Proctor GB, Ebersole LE, et al. Secretion of IgA by rat parotid and submandibular cells in response to autonomimetic stimulation in vitro. Int. Immunopharmacol. 2004;4:1005–1014.10.1016/j.intimp.2004.03.013
- Chicharro JL, Lucia A, Perez M, et al. Saliva composition and exercise. Sports Med. 1998;26:17–27.10.2165/00007256-199826010-00002
- Klentrou P, Cieslak T, MacNeil M, et al. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur. J. Appl. Physiol. 2002;87:153–158.10.1007/s00421-002-0609-1
- Tiollier E, Gomez-Merino D, Burnat P, et al. Intense training: mucosal immunity and incidence of respiratory infections. Eur. J. Appl. Physiol. 2005;93:421–428.10.1007/s00421-004-1231-1
- Ronsen O, Pedersen BK, Øritsland TR, et al. Leukocyte counts and lymphocyte responsiveness associated with repeated bouts of strenuous endurance exercise. J. Appl. Physiol (1985). 2001;91:425–434.
- Gleeson M, Pyne DB, Austin JP, et al. Epstein-Barr virus reactivation and upper-respiratory illness in elite swimmers. Med. Sci. Sports. Exerc. 2002;34:411–417.10.1097/00005768-200203000-00005
- Castell L. Glutamine supplementation in vitro and in vivo, in exercise and in immunodepression. Sports Med. 2003;33:323–345.10.2165/00007256-200333050-00001
- Mackinnon LT, Ginn E, Seymour GJ. Decreased salivary immunoglobulin A secretion rate after intense interval exercise in elite kayakers. Eur. J. Appl. Physiol. Occup. Physiol. 1993;67:180–184.10.1007/BF00376664
- Nieman DC, Henson DA, Fagoaga OR, et al. Change in salivary IgA following a competitive marathon race. Int. J. Sports. Med. 2002;23:69–75.10.1055/s-2002-19375
- Novas AM, Rowbottom DG, Jenkins DG. Tennis, incidence of URTI and salivary IgA. Int. J. Sports. Med. 2003;24:223–229.10.1055/s-2003-39096
- Kimura F, Aizawa K, Tanabe K, et al. A rat model of saliva secretory immunoglobulin: a suppression caused by intense exercise. Scand. J. Med. Sci. Sports. 2008;18:367–372.10.1111/sms.2008.18.issue-3
- Gholamnezhad Z, Boskabady MH, Hosseini M, et al. Evaluation of immune response after moderate and overtraining exercise in wistar rat. Iran. J. Basic. Med. Sci. 2014;17:1–8.
- Shibuya T, Kaburagi T, Nagai R, et al. The effects of moderate exercise on secretory IgA production in mice depends on dietary carbohydrate intake. J. Clin. Biochem. Nutr. 2015;57:44–49.10.3164/jcbn.15-21
- Moraska A, Deak T, Spencer RL, et al. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1321–1329.
- Morifuji M, Sanbongi C, Sugiura K. Dietary soya protein intake and exercise training have an additive effect on skeletal muscle fatty acid oxidation enzyme activities and mRNA levels in rats. Br. J. Nutr. 2006;96:469–475.
- Matsuo T, Nozaki T, Okamura K, et al. Effects of voluntary resistance exercise and high-protein snack on bone mass, composition, and strength in rats given glucocorticoid injections. Biosci. Biotechnol. Biochem. 2003;67:2518–2523.10.1271/bbb.67.2518
- Lee S, Barton ER, Sweeney HL, et al. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J. Appl. Physiol (1985). 2004;96:1097–1104.
- Matsumoto M, Inoue R, Tsukahara T, et al. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol Biochem. 2008;72:572–576.10.1271/bbb.70474
- Holy X, Zérath E. Bone mass increases in less than 4 wk of voluntary exercising in growing rats. Med. Sci. Sports. Exerc. 2000;32:1562–1569.10.1097/00005768-200009000-00006
- Yamamoto Y, To M, Hayashi T, et al. Intake of indigestible carbohydrates influences IgA response and polymeric Ig receptor expression in the rat submandibular gland. Br. J. Nutr. 2015;113:1895–1902.10.1017/S0007114515001403
- Quadrilatero J, Hoffman-Goetz L. Physical activity and colon cancer. A systematic review of potential mechanisms. J. Sports Med. Phys. Fitness. 2003;43:121–138.
- Suzuki K, Totsuka M, Nakaji S, et al. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol (1985). 1999;87:1360–1367.
- Suzuki K, Nakaji S, Yamada M, et al. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc. Immunol. Rev. 2002;8:6–48.
- Pedersen BK, Steensberg A, Keller P, et al. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. 2003;446:9–16.10.1007/s00424-002-0981-z
- Allen JM, Berg Miller ME, Pence BD, et al. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6 J mice. J. Appl. Physiol (1985). 2015;118:1059–1066.
- Holloszy JO. Exercise increases average longevity of female rats despite increased food intake and no growth retardation. J. Gerontol. 1993;48:B97–100.10.1093/geronj/48.3.B97
- Hall-López J, Ochoa-Martínez P, Teixeira AM, et al. Effect of hydrogymnastics physical exercise on serum level of immunoglobulin A in elderly women. Rev. Chilena Infectol. 2015;32:272–277.10.4067/S0716-10182015000400003
- Kvetnanský R, Pacák K, Fukuhara K, et al. Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann. N. Y. Acad. Sci. 1995;771:131–158.10.1111/nyas.1995.771.issue-1
- Droste SK, Chandramohan Y, Hill LE, et al. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37.10.1159/000104770
- Bruunsgaard H, Galbo H, Halkjaer-Kristensen J, et al. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J. Physiol. 1997;499:833–841.10.1113/jphysiol.1997.sp021972
- Cannon JG, Fiatarone MA, Fielding RA, et al. Aging and stress-induced changes in complement activation and neutrophil mobilization. J. Appl. Physiol (1985). 1994;76:2616–2620.
- Lancaster GI, Khan Q, Drysdale PT, et al. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J. Appl. Physiol (1985). 2005;98:565–571.
- Zaldivar F, Wang-Rodriguez J, Nemet D, et al. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J. Appl. Physiol (1985). 2006;100:1124–1133.
- Street NE, Mosmann TR. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991;5:171–177.
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173.10.1146/annurev.iy.07.040189.001045
- McGhee JR, Mestecky J, Elson CO, et al. Regulation of IgA synthesis and immune response by T cells and interleukins. J. Clin. Immunol. 1989;9:175–199.10.1007/BF00916814
- Loman S, Jansen HM, Out TA, et al. Interleukin-4 and interferon-gamma synergistically increase secretory component gene expression, but are additive in stimulating secretory immunoglobulin A release by Calu-3 airway epithelial cells. Immunology. 1999;96:537–543.10.1046/j.1365-2567.1999.00731.x