Abstract
Fecal and blood samples of infants with autism spectrum disorders (ASD) and healthy infants were analyzed to investigate the association of altered gut microbiota and ASD development. 16S rRNA gene-based sequencing found that, unlike those of healthy infants, feces of ASD infants had significantly higher and lower abundance of genera Faecalibacterium and Blautia, respectively. Moreover, DNA microarray analysis of peripheral blood mononuclear cells (PBMC) detected more highly than low expressed genes in ASD infants than in healthy infants. Gene Ontology analysis revealed that differentially expressed genes between ASD and healthy infants were involved in interferon (IFN)-γ and type-I IFN signaling pathways. Finally, strong positive correlations between expression of IFN signaling-associated genes in PBMC and fecal abundance of Faecalibacterium were found. Our results strongly suggested that altered gut microbiota in infants resulted from ASD development and was associated with systemic immunity dysregulation, especially chronic inflammation.
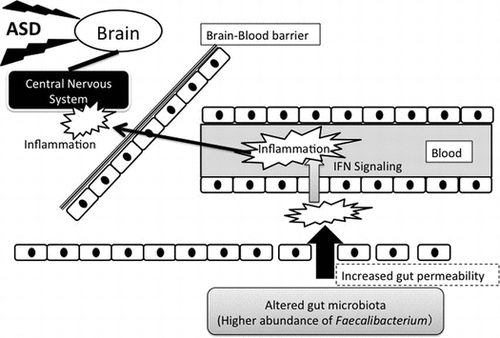
Graphical abstract
Altered gut microbiota in autistic infants play a role in chronic inflammation induced by interferon signaling that may cause further inflammation in central nervous system.
Autism spectrum disorders (ASD) are complex neurophysiological disorders characterized by reduced qualitative social and communication skills but augmented ritualistic and stereotyped behavior.Citation1) Extensive immune and neuronal dysregulation has been suggested to be involved in the development of ASD but the underlying etiology is not yet well understood.Citation1) Recently, a greater number of ASD cases have been reported, but this increase cannot be explained only by greater awareness and improved diagnosis methods. Thus, it is likely that environmental factorsCitation2) as well as geneticsCitation3) play a role in the development of ASD.
Gut microbiota, consisting of more than 500 microbial species,Citation4) has lately attracted attention concerning the onset of ASD. Indeed, several studies reported that disruption of the gut microbiota was more frequent in individuals with ASD than it was in healthy individuals.Citation5–12) Emerging evidence shows that the gut microbiota affects not only nutritional and immunological functionsCitation13) but also neural signalingCitation14) and behavioral traits.Citation15) Hence, it is very likely that the gut microbiota plays an important role in the development of ASD.
It remains unclear, however, whether altered gut microbiota is indeed a causative or deteriorating factor in ASD, or only a consequence of the disease, because no consistent trend of abnormality in the gut microbiota of individuals with ASD has been established yet. In an elegant summary of work on the association of the gut microbiota with individuals with ASD, LouisCitation16) highlighted several inconsistencies among major studies. For example, a wide variation in bacterial diversity in general, and Bacteriodetes proportions in particular, was reported across studies.Citation16) More recently, several other studies have reported altered gut microbiota in individuals with ASD,Citation17,18) but their data still lacked consistency with previous studies.
Due to the conflicting data currently available, there is clearly a need for further investigation of the relationship between the gut microbiota and ASD. To that end, in the present work we compared the gut microbiota composition in infants with ASD with that in healthy infants because no such study has been conducted in Asia. Furthermore, we analyzed the transcriptome of peripheral blood mononuclear cells (PBMC) from the same infants as an innovative component. In addition, we evaluated whether genes expressed at different degrees in PBMC of infants with ASD correlated with the abundance of a particular gut bacterial genus in fecal microbiota. Our results strongly suggested that altered gut microbiota in infants with ASD was not merely a consequence of the disease, but was also associated with dysregulation of systemic immunity, especially chronic inflammation in ASD.
Materials and methods
Ethics statements
The present work conformed to the code of ethics stated in the Declaration of Helsinki. The research protocol was approved by the Ethical Committee of Shiga University of Medical Science (Shiga, Japan; Approval number: 24-161). Written informed consent was obtained from the parents of the infant donors to use the fecal and blood samples for research purposes. The privacy rights of the infant donors were observed at all times.
Subjects
Six infants with ASD (experiment group) and six healthy infants (control group) in the range of 3–5 years of age and living in similar geographic regions in Japan were enrolled in this study. The infants in the ASD group were pre-categorized by the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria and diagnosed according to the Pervasive Developmental Disorders Autism Society Japan Rating Scale (PARS)Citation19) and Modified Checklist for Autism in Toddlers (M-CHAT).Citation20) No infant had a considerable gut disorder or took antibiotics or drugs to treat ASD for at least one month before the sampling period.
Collection of feces and extraction of bacterial genomic DNA
Freshly evacuated feces were aseptically collected, placed into sterilized containers (Stool Carry, Atect, Osaka, Japan) and kept at −20 °C. Containers containing the feces were transported to a laboratory within 24 h post-evacuation and stored at −80 °C until further use. Bacterial genomic DNA was extracted from the fecal samples as previously described.Citation21)
Library preparation and DNA sequencing
Preparation of library for DNA sequencing by a Miseq desktop sequencer (Illumina, San Diego, CA, USA) was carried out as per a protocol by Illumina (https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf) with minor modifications. Briefly, the V3-4 region of 16S rRNA genes in each sample was amplified by primers 341F and 805R containing 5′ overhang adapter sequence for PCR using a KAPA HiFi HotStart Ready Mix (Kapa Biosciences, Wilmington, MA, USA). The amplicon was purified by NucleoFast 96 PCR plates (Takara Bio Inc., Shiga, Japan). A second PCR was carried out using the KAPA HiFi HotStart Ready Mix (Kapa Biosciences) to attach a unique combination of dual indices (I5 and I7 index) and Illumina sequencing adapters to each sample. The amplicon of the second PCR was purified and the concentration was normalized with a SequalPrep Normalization Plate Kit (Life Technologies, Tokyo, Japan). Each of the normalized amplicons was then evenly pooled and concentrated using AMPure XP beads (Beckman Coulter, Tokyo, Japan). The size and quantity of the library were assessed with a Bioanalyzer 2100 (Agilent Technologies Japan, Tokyo, Japan) and a Library Quantification Kit for Illumina (Kapa Biosciences), respectively. The library was denatured with 0.2 N NaOH (Sigma-Aldrich, Tokyo, Japan) and combined with phiX Control (v3, Illumina; expected 20%). Eleven pM of the library combined with phiX Control was heat-denatured at 96 °C for 2 min and sequenced using a 300-bp paired-end strategy on the Miseq (Illumina) as per the manufacturer’s instructions.
Sequence data analysis
Sequences were first processed according to Fadrosh et al.Citation22) using a modified version of the original script kindly donated by Dr B. Ma and Prof J. Ravel of the University of Maryland School of Medicine. Briefly, 8-bp index sequences at both ends extracted by Miseq Reporter software (v2.4.60) were concatenated to form a 16-bp dual-index barcode specific for each paired-end read. Next, each dual-index barcode was paired with the corresponding sample sequence that was assembled with Fast Length Adjustment of Short reads (FLASH) v1.2.11.Citation23) De-multiplexing of the sequences according to the dual-index was carried out by Quantitative Insights Into Microbial Ecology (QIIME) v1.9.0Citation24), allowing a Phred score higher than Q21, a maximum of 3 errors in barcodes and no Ns. Afterwards, sequence analysis tool USEARCH v8.0Citation25) was used for further filtering (maximum expected error = 0.5), dereplication, discarding clusters less than 5 reads, and clustering OTU. Chimera was validated and removed with UCHIME version 4.2.40Citation26) in reference mode using the Broad Institute’s 16S rRNA gene Gold reference database. Taxonomy assignment of resulting OTU was carried out using RDP classifier v2.10.2Citation27) with the Greengenes database (published May, 2013). Default settings were used for all software unless otherwise stated.
Collection of whole blood and isolation of PBMC
Peripheral blood was drawn from four of the six infants with ASD (A2-A5) and four of the six healthy infants (C2, C4-C6), and collected in Venoject II vacuum blood collection tubes (heparinized blood collection tubes) (Terumo Medical Corp., Tokyo, Japan). PBMC were isolated as previously described by Nishibayashi et al.Citation28)
Microarray analysis of PBMC
Total RNA was extracted and cDNA synthesized from 35 ng of total RNA as described by Ogawa et al.Citation29) Complementary DNA was labeled with Cy3-dUTP (GE-Healthcare, Tokyo, Japan) with a random primer (Life Technologies) using 50 units of Klenow Polymerase (New England Biolabs, Tokyo, Japan) in the presence of 0.5 mM dATP, dGTP and dCTP, and 0.05 mM dTTP. After 3-h incubation at 37 °C, Cy3-labeled cDNA was purified with a PureLink Quick PCR Purification kit (Life Technologies). The quantity and quality of Cy3-labeled cDNA were verified using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Six hundred ng of each Cy3-labeled cDNA sample was fragmented by incubating with 0.55 Kunitz unit of DNase I (RNase-Free DNase Set; Qiagen) for 8 min at 37 °C. This incubation time was determined in advance so that the size of cDNA became approximately 50–150 bases. DNase I was inactivated for 2 min at 98 °C. Samples were then hybridized to the array slide [SurePrint G3 Human GE microarray, 8 × 60 K, V2; Agilent Technologies Japan] for 16 h at 65 °C. The array was washed as per the manufacturer’s instructions and scanned with an Agilent G2565CA microarray scanner. Intensity data were extracted using Feature Extraction software (Agilent).
Real-time RT-PCR
The expression of IFN-related genes (IRF7, IRF9, CXCL10 and CXCL11) in PBMC was analyzed by real-time RT-PCR. Complementary DNA was synthesized from each total RNA sample (20 ng) using a RevaTra Ace qPCR RT kit (TOYOBO, Osaka, Japan), as per the manufacturer’s instructions. Real-time PCR was conducted using a LightCycler 480 instrument (Roche Applied Science, Tokyo, Japan). Specific primers and probe sets for each gene were designed with online software available on the Universal ProbeLibrary Assay Design Center (Roche Applied Science). Sequences of the primers and probes used in this study are shown in Table S1. GAPDH and ACTB were used as the reference genes, and PCR conditions and analyses conducted for all genes were as described by Yoshikawa et al.Citation30) The analysis was separately conducted twice.
Data analysis
The abundance (percentage) of each bacterial genus between groups was compared by the Student’s t-test. The Student t-test was performed by Excel® (Microsoft Corp., Redmond, WA, USA).
Intensity data from the microarray analysis were analyzed by CLC MainWork bench v6.7 (CLC bio Japan, Tokyo, Japan). Signal intensity values were normalized by the quartile normalization method and log2 transformed. The Student’s t-test was used to evaluate differences in the transformed values of each microarray probe between groups. The p value and the fold difference of each gene were imported into web-based pathway analysis software (iPathway), and the Gene Ontology (GO) analysis was conducted on the differentially expressed genes (−1.5 ≤ fold change ≥ 1.5, p < 0.05) to detect the processes in which they were involved. Afterwards, the correlation between the expression level (transformed value) of the differentially expressed genes involved in the GO biological processes and the abundance of bacterial genera in fecal microbiota that was significantly different between groups were assessed with the Pearson’s product-moment coefficient using freely available R software v3.1.2.
For the real-time RT-PCR analysis, the gene expression level relative to the expression level of the C2 subject was calculated by the Delta Ct method and correlation between this expression value and the abundance of Faecalibacterium in fecal microbiota was assessed as described above. The Delta Ct value was also used for the Student’s t-test analysis of the comparison between groups.
Results
Fecal microbiota in ASD infants and healthy infants
A total of 307,022 sequence reads were obtained after data processing (average 25,585.2 reads; ranged 10,344–37,647). The abundance of genera Faecalibacterium and Blautia was significantly different between infants with ASD and healthy infants (Table ). The abundance of genus Faecalibacterium was significantly higher in the fecal microbiota of ASD infants (9.78 ± 6.75%) than it was in that of healthy infants (3.25 ± 1.61%). In contrast, the abundance of genus Blautia was significantly lower in the fecal microbiota of ASD infants (3.34 ± 4.50%) than it was in that of healthy infants (14.21 ± 10.02%).
Table 1. Relative abundance (%) of bacterial family/genera in the fecal microbiota of ASD infants and healthy infants.
Differentially expressed genes in PBMC between infants with ASD and healthy infants
There were 1,056 more highly expressed genes in ASD infants than in healthy infants. In contrast, expression of 517 more genes was significantly low in ASD infants than they were in healthy infants.
With regard to the GO analysis of the differentially expressed genes, a considerable number of GO biological processes associated with response to viruses (six of 20) were enriched. The top 20 GO biological processes significantly enriched are listed in Table . Processes apart from the top 20 were further analyzed to detect processes associated with interferon, as some studies reported an increase in interferon (IFN)-α or IFN-γ in infants with ASD.Citation31–33) Three and five processes associated with IFN-γ and type I IFN, respectively, were found to be significantly enriched (Table ). Furthermore, interferon regulatory factor (IRF)7 and IRF9 genes were involved in seven of the eight processes associated with interferon.
Table 2. GO pathways enriched for the differentially expressed genes between ASD infants and healthy infants.
Table 3. Interferon-related GO pathways enriched for the differentially expressed genes between ASD infants and healthy infants.
Correlation between differentially expressed genes in PBMC and the abundance of bacterial genera in fecal microbiota
Three and two of eight differentially expressed genes involved in the GO process named IFN-γ-mediated signaling pathway (GO:0060333) significantly correlated with the abundance of Faecalibacterium and Blautia, respectively (Table ). In addition, three of eight differentially expressed genes showed a correlation that tended to be significant (p < 0.1) with either genus (Table ). Interestingly, although six of seven genes involved in the GO process named type I interferon signaling pathway (GO:0060337) significantly correlated with the abundance of genus Faecalibacterium, only IRF7 correlated with the abundance of Blautia (Table ).
Table 4. Correlation between the expression level of genes involved in interferon signaling pathways in PBMC and the abundance of Faecalibacterium and Blautia in fecal microbiota.
Real-time PCR analysis
Normalization by housekeeping genes GAPDH and ACTB yielded very similar data. Thus, in the present work only data normalized by GAPDH are shown.
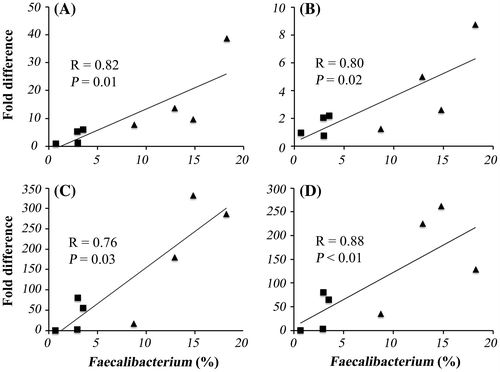
The expression of all IFN signaling-associated genes evaluated by real-time RT-PCR was higher in ASD infants than it was in healthy infants, and exactly as it was detected by the microarray analysis. IRF7 was expressed significantly higher in ASD infants than it was in healthy infants, and the expression of three other genes tended to be higher also in ASD infants (p < 0.1). Finally, the expression of all genes significantly correlated with the abundance of genus Faecalibacterium (Fig. ).
Fig. 1. Correlation of gene expression levels in PBMC with the abundance of genus Faecalibacterium in fecal microbiota.
Discussion
ASD are putatively caused by multiple genetic and non-genetic etiologies.Citation1) Etiological differences between ASD infants and healthy infants have been evaluated from various aspects including genetic mutations,Citation3) brain activity,Citation34) neuronalCitation33) and immune functions,Citation35) metabolismCitation36) and gut microbiota composition.Citation16) In this study, we evaluated the differences between ASD infants and healthy infants by focusing on the gut microbiota and its relation with transcriptomic profiles of PBMC, namely, genes involved in interferon signaling.
The number of samples from ASD infants used in the present work may seem to be relatively small in comparison with the numbers usually reported in clinical trials. The reason for the small number of samples was due to the fact that consent from Japanese parents of autistic infants to analyze their fecal and peripheral blood samples was difficult to obtain. We attribute this parental unwillingness to participate to inherent social and cultural characteristics of Japanese society. For example, in Japan, emotional and mental problems are still stigmatized.Citation37) As a result, these problems are frequently hidden from and denied to outsiders.Citation38) Nonetheless, we are confident that, although preliminary, the data obtained from statistically significant correlations between the Faecalibacterium abundance and gene expression levels in infant fecal and blood samples, respectively, were sufficiently solid to support our conclusions.
The abundance of two bacterial genera in fecal microbiota was significantly different between ASD infants and healthy infants. Furthermore, compared with healthy infants, ASD infants had a significantly higher abundance of genus Faecalibacterium, but a significantly lower abundance of genus Blautia. Faecalibacterium prausnitzii is the only species known to belong to genus FaecalibacteriumCitation39) and thus, has been mostly regarded as commensal or even beneficial.Citation40) Moreover, recent studies showed that this species produces microbial anti-inflammatory molecule, an anti-inflammatory protein,Citation41,42) and that a decrease of F. prausnitzii is associated with human inflammatory disorders.Citation40) Thus, a high population of this genus in fecal samples of ASD infants in the present study was unexpected. Nonetheless, this genus seems to be not always beneficial, especially in infants. For example, Hansen et al.Citation43) found that F. prausnitzii was more abundant in infants with Crohn’s disease than it was in control infants, and Balamurugan et al.Citation44) reported a higher number of F. prausnitzii in obese infants than in infants with normal weight. Gut inflammation is a symptom often observed in experimental models of obesity.Citation45) Recently, Song et al.Citation46) also found an enrichment of F. prausnitzii and a reduced number of anti-inflammatory effect-inducing bacteria in the fecal samples of patients suffering from atopic dermatitis, a chronic skin inflammation disease. Therefore, it is likely that higher abundance of Faecalibacterium in ASD infants in this study may have resulted in gut inflammation, at least to some extent.
It is important to mention that the evidence of abnormality detected in the gut microbiota of ASD infants in this study was also inconsistent with previous human studies investigating the gut microbiota in ASD infantsCitation5–12,17,18) and even in a mouse model.Citation47) For example, no studies have suggested an increase in Faecalibacterium abundance in ASD infants. In addition, the genus Blautia consists of bacteria re-clustered from Clostriudium cluster XIVa,Citation48) and no relation linking this bacterial group with ASD has been reported yet. Hence, to investigate whether abnormalities in the gut microbiota were plausibly involved in the etiologies of ASD or only a consequence of biased eating habits and food preferences often reported in ASD infants,Citation49) we evaluated the correlation between the expression levels of the differentially expressed genes in PBMC and the abundance of Faecalibacterium and Blautia.
We examined the correlated genes by focusing on IFN-related signaling processes enriched by the GO analysis, and found that, unlike Blautia abundance, Faecalibacterium abundance was strongly correlated with a greater number of differentially expressed genes involved in both the interferon-γ-mediated signaling pathway and the type I interferon signaling pathway, but in particular in the latter. Although conflicting reports exist,Citation50,51) a higher concentration of IFN-α or IFN-γ in cerebrospinal fluid or plasma/serum and higher production of IFN-γ by blood cells have been reported in several studies.Citation31–33) It is also of particular interest that large doses of IFN-α given to children in cancer treatment cause similar symptoms to those of autism, namely, withdrawing, lost interest in their surroundings and non-communication.Citation52)
To further confirm the correlation between Faecalibacterium abundance in fecal microbiota and the genes associated with IFN signaling, the expression level of IRF7 and IRF9 was measured by real-time PCR as they would be plausible markers for activation of type I interferon signaling.Citation53,54) Genes CXCL10 and CXCL11 were also evaluated because they were (a) induced by interferon signalingCitation55–57) and, (b) expressed higher than 4-fold in ASD infants than they were in healthy infants in microarray analyses (although this expression was not statistically significant). In addition, an elevated concentration of CXCL10 or CXCL11, but especially the former, has been previously reported in serum or cerebrospinal fluid of individuals with several neuroimmunological disorders, including ASD.Citation33,58–60) The expression level of IRF7 and IRF9 evaluated by real-time PCR showed a strong positive correlation with the abundance of Faecalibacterium in fecal microbiota, which was consistent with our results from the microarray analysis. Moreover, the expression level of CXCL10 and CXCL11 also significantly correlated with Faecalibacterium abundance. Accordingly, the expression of all genes evaluated by real-time PCR was higher in PBMC of ASD infants than it was in those of healthy infants.
Taken all the results together, three scenarios can be suggested. First, as suggested in previous studies,Citation61) immune dysfunction may be a common feature in ASD infants and is likely associated with chronic inflammation because IFN has been suggested to be involved in chronic, not acute inflammation.Citation62,63) Second, the gut microbiota of ASD infants may be typically different than that of healthy infants of same age, because unlike healthy infants, ASD infants in the present study had high and low abundance of genera Faecalibacterium and Blautia, respectively. Third, the abundance of Faecalibacterium and Blautia, especially the former, may play a role in the dysfunction of systemic immunity as their abundance in fecal microbiota significantly correlated with the expression level of many genes in PBMC, including the genes associated with IFN signaling.
As suggested by other studies,Citation64) increased gut permeability is likely involved in the mechanism by which abnormal gut microbiota affects gene expression in PBMC and consequently induces systemic immune dysfunctions, but further investigation of this mechanism was beyond the scope of this study. Nonetheless, it can be theorized that high abundance of Faecalibacterium in the gut microbiota of infants may indicate a mild to moderate gut inflammation, and this condition may be indicative of increased gut permeability as well.Citation65) Furthermore, increased gut permeability reportedly results in higher antigenic load,Citation64) which may have induced dysfunction of systemic immunity including an inflammatory response of PBMC.
Conclusion
In conclusion, in the present study, we demonstrated that alterations of the gut microbiota play an important role in the dysfunction of systemic immunity and thus the gut microbiota could be at least considered a deteriorating factor of ASD. Although the number of infants evaluated in this study was relatively small, all statistically significant correlations found between the abundance of Faecalibacterium and gene expression levels in PBMC strongly support our conclusions. Further study is required to reveal the exact link between an abnormal gut microbiota and ASD, and the effects caused by abnormal gut microbiota on infants with ASD.
Author contributions
R.I., Y.S., C.S., T.S., and T.T. designed research, Y.S., and C.S. collected clinical samples, R.I., and T.T. performed the experiments, R.I., and M.O. analyzed data, and R.I., and G.A.R. wrote the manuscript. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
This study was supported in part by The Morinaga Foundation for Health & Nutrition.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1222267.
TBBB_1222267_Supplementary_Material.docx
Download MS Word (21.1 KB)Acknowledgment
The authors thank Dr T. Tsuruta, R. Nishibayashi and M. Nakatani at Kyoto Prefectural University for their technical assistance. The authors would also like to thank Drs B. Ma and M. Humphreys and Prof J. Ravel of the University of Maryland School of Medicine for their assistance in the analysis of sequencing data.
References
- Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910.10.1016/S0140-6736(13)61539-1
- Dietert RR, Dietert JM, Dewitt JC. Environmental risk factors for autism. Emerg. Health Threats J. 2011;4:7111.
- Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat. Rev. Neurol. 2014;10:74–81.10.1038/nrneurol.2013.278
- Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014;10:416–424.10.1038/nchembio.1535
- Adams JB, Johansen LJ, Powell LD, et al. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22.10.1186/1471-230X-11-22
- Finegold SM, Dowd SE, Gontcharova V, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453.10.1016/j.anaerobe.2010.06.008
- Finegold SM, Molitoris D, Song Y, et al. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 2002;35:S6–S16.10.1086/cid.2002.35.issue-s1
- Parracho HM, Bingham MO, Gibson GR, et al. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005;54:987–991.10.1099/jmm.0.46101-0
- Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004;70:6459–6465.10.1128/AEM.70.11.6459-6465.2004
- Wang L, Christophersen CT, Sorich MJ, et al. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 2011;77:6718–6721.10.1128/AEM.05212-11
- Williams BL, Hornig M, Buie T, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585.10.1371/journal.pone.0024585
- Williams BL, Hornig M, Parekh T, et al. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012;3:e00261-1–e00261-11.
- Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336.10.1038/nature10213
- Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014.10.1053/j.gastro.2009.01.075
- Neufeld KM, Kang N, Bienenstock J, et al. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23:255–264, e119.
- Louis P. Does the human gut microbiota contribute to the etiology of autism spectrum disorders? Dig. Dis. Sci. 2012;57:1987–1989.10.1007/s10620-012-2286-1
- De Angelis M, Piccolo M, Vannini L, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8:e76993.10.1371/journal.pone.0076993
- Kang DW, Park JG, Ilhan ZE, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322.10.1371/journal.pone.0068322
- Kamio Y, Yukihiro R, Adachi J, et al. Reliability and validity of the pervasive developmental disorder (PDD)-Autism society Japan rating scale (PARS): a behavior checklist for adolescents and adults with PDDs. Clin. Psychiat. (Seishin-Igaku). 2006;48:495–505.
- Robins DL, Fein D, Barton ML, et al. The modified checklist for autism in toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J. Autism Dev. Disord. 2001;31:131–144.10.1023/A:1010738829569
- Matsumoto M, Inoue R, Tsuruta T, et al. Long-term oral administration of cows’ milk improves insulin sensitivity in rats fed a high-sucrose diet. Br. J. Nutr. 2009;102:1324–1333.10.1017/S0007114509990365
- Fadrosh DW, Ma B, Gajer P, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2:6.10.1186/2049-2618-2-6
- Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963.10.1093/bioinformatics/btr507
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336.10.1038/nmeth.f.303
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461.10.1093/bioinformatics/btq461
- Edgar RC, Haas BJ, Clemente JC, et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200.10.1093/bioinformatics/btr381
- Wang Q, Garrity GM, Tiedje JM, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267.10.1128/AEM.00062-07
- Nishibayashi R, Inoue R, Harada Y, et al. RNA of Enterococcus faecalis strain EC-12 is a major component inducing interleukin-12 production from human monocytic cells. PLoS One. 2015;10:e0129806.10.1371/journal.pone.0129806
- Ogawa S, Okutani M, Tsukahara T, et al. Comparison of gene expression profiles of T cells in porcine colostrum and peripheral blood. Am. J. Vet. Res. Forthcoming.
- Yoshikawa T, Inoue R, Matsumoto M, et al. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem. Cell Biol. 2011;135:183–194.10.1007/s00418-011-0779-1
- Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J. Neuroimmunol. 1996;66:143–145.
- Stubbs G. Interferonemia and autism. J. Autism Dev. Disord. 1995;25:71–73.10.1007/BF02178169
- Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005;57:67–81.10.1002/(ISSN)1531-8249
- Paakki JJ, Rahko J, Long X, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010;1321:169–179.10.1016/j.brainres.2009.12.081
- Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci. 2015;16:469–486.10.1038/nrn3978
- Bell JG, MacKinlay EE, Dick JR, et al. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukot. Essent. Fatty Acids. 2004;71:201–204.10.1016/j.plefa.2004.03.008
- Shon SP, Ja DA. Ethnicity and family therapy. In: McGoldrick M, Pearce JK, and Giordano J, editors. New York, NY: The Guilford Press; 1982. p. 208–228.
- Braun KL, Browne CV. Perceptions of dementia, caregiving, and help seeking among Asian and Pacific Islander Americans. Health Soc. Work. 1998;23:262–274.10.1093/hsw/23.4.262
- Duncan SH, Hold GL, Harmsen HJ, et al. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002;52:2141–2146.
- Miquel S, Martin R, Rossi O, et al. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013;16:255–261.10.1016/j.mib.2013.06.003
- Quévrain E, Maubert MA, Michon C, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65:415–425.10.1136/gutjnl-2014-307649
- Quévrain E, Maubert M-A, Sokol H, et al. The presence of the anti-inflammatory protein MAM, from Faecalibacterium prausnitzii, in the intestinal ecosystem. Gut. 2016;65:882.
- Hansen R, Russell RK, Reiff C, et al. Microbiota of de-novo pediatric IBD: increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn’s but not in ulcerative colitis. Am. J. Gastroenterol. 2012;107:1913–1922.10.1038/ajg.2012.335
- Balamurugan R, George G, Kabeerdoss J, et al. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br. J. Nutr. 2010;103:335–338.10.1017/S0007114509992182
- de La Serre CB, Ellis CL, Lee J, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G440–G448.10.1152/ajpgi.00098.2010
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2015;137:852–860.
- Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463.10.1016/j.cell.2013.11.024
- Liu C, Finegold SM, Song Y, et al. Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov. and description of Blautia wexlerae sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2008;58:1896–1902.10.1099/ijs.0.65208-0
- Schreck KA, Williams K, Smith AF. A comparison of eating behaviors between children with and without autism. J. Autism Dev. Disord. 2004;34:433–438.10.1023/B:JADD.0000037419.78531.86
- Ashwood P, Krakowiak P, Hertz-Picciotto I, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011;25:40–45.10.1016/j.bbi.2010.08.003
- Suzuki K, Matsuzaki H, Iwata K, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One. 2011;6:e20470.10.1371/journal.pone.0020470
- Hill NO, Pardue A, Kahn A, et al. Phase 1 human leukocyte interferon trials in cancer and leukemia. J. Clin. Hematol. Oncol. 1981;11:23–35.
- Finsen B, Owens T. Innate immune responses in central nervous system inflammation. FEBS Lett. 2011;585:3806–3812.10.1016/j.febslet.2011.05.030
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5:375–386.10.1038/nri1604
- Müller A, Oertli M, Arnold IC. H. pylori exploits and manipulates innate and adaptive immune cell signaling pathways to establish persistent infection. Cell Commun. Signal. 2011;9:25.10.1186/1478-811X-9-25
- Takayanagi H. All interferons are not equal: specific mechanisms of interfering with osteoclastogenesis. Bonekey Osteovision. 2005;2:24–28.10.1138/20050183
- Mehla R, Guha D, Ayyavoo V. Chemokine deregulation in HIV infection: role of interferon gamma induced Th1-chemokine signaling. J. Clin. Cell Immunol. 2012;S7:4.
- Lepej SŽ, Mišić-Majerus L, Jeren T, et al. Chemokines CXCL10 and CXCL11 in the cerebrospinal fluid of patients with tick-borne encephalitis. Acta Neurol. Scand. 2007;115:109–114.10.1111/ane.2007.115.issue-2
- Moniuszko A, Czupryna P, Pancewicz S, et al. Evaluation of CXCL8, CXCL10, CXCL11, CXCL12 and CXCL13 in serum and cerebrospinal fluid of patients with neuroborreliosis. Immunol. Lett. 2014;157:45–50.10.1016/j.imlet.2013.11.002
- Müller M, Carter S, Hofer MJ, et al. Review: the chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity – a tale of conflict and conundrum. Neuropathol. Appl. Neurobiol. 2010;36:368–387.10.1111/nan.2010.36.issue-5
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J. Leukoc. Biol. 2006;80:1–15.
- Akbar AN, Lord JM, Salmon M. IFN-α and IFN-β: a link between immune memory and chronic inflammation. Immunol. Today. 2000;21:337–342.10.1016/S0167-5699(00)01652-2
- Lee PY, Li Y, Kumagai Y, et al. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am. J. Pathol. 2009;175:2023–2033.10.2353/ajpath.2009.090328
- de Theije CG, Wu J, da Silva SL, et al. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur. J. Pharmacol. 2011;668(Suppl 1):S70–S80.10.1016/j.ejphar.2011.07.013
- Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520.
