Abstract
Plant chitinases play diverse roles including defense against pathogenic fungi. Using reverse-transcription quantitative PCR analysis, we found that six chitinase (PpChi) genes and two genes for chitin elicitor receptor kinases (PpCERKs) are expressed at considerable levels in the moss Physcomitrella patens subsp. patens. The expressed PpChis belonged to glycoside hydrolase family 19 (class I: PpChi-Ia and -Ib; class II: PpChi-IIa and -IIc; and class IV: PpChi-IV) and to glycoside hydrolase family 18 (class V: PpChi-Vb). Treatment with chitin tetramer or hexamer increased the expression of class I and IV PpChi genes and decreased that of class II PpChi genes. Recombinant PpChi-Ia, PpChi-IV, and PpChi-Vb were characterized. PpChi-IV exhibited higher activity against chitin tetramer and pentamer than PpChi-Ia did. PpChi-Vb showed transglycosylation activity and PpChi-Ia inhibited fungal growth. These results suggest that chitinases of different classes play different roles in defense mechanism of moss plant against fungal pathogens.
Graphical abstract
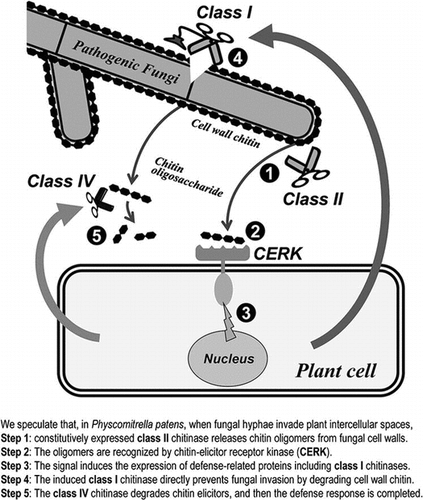
Possible roles of each class of chitinase and chitin elicitor receptor kinase (CERK) for self-defense against pathogenic fungi in moss plant.
Chitinases (EC 3.2.1.14) catalyze the hydrolysis of chitin, a β-1,4-linked homopolymer or oligomer of N-acetylglucosamine (GlcNAc). Most plants have chitinases, which play diverse roles in plant as reviewed by Kasprzewska.Citation1) However, their endogenous substrate in plants has not yet been found.Citation2,3) In the absence of an endogenous substrate, some of the plant chitinases may be involved in the interaction between plants and microbes, which produce chitin and chitin-related compounds. It was reported that one of the physiological roles of these chitinases is to protect plants from fungal pathogens by degrading chitin, a major component of the cell wall of many fungi.Citation4,5) Gene expression and activity of chitinases are increased considerably upon infection with pathogenic fungi, and chitinase overexpression enhances host plant resistance against pathogenic fungi.Citation2,3,6)
According to the CAZy database (http://www.cazy.org/), plant chitinases are divided into the glycoside hydrolase family 18 (GH18) or 19 (GH19) on the basis of the structure of their catalytic domains. The catalytic domains of plant chitinases of classes I, II, II-L, and IV belong to GH19, whereas those of classes III, IIIb, and V belong to GH18.Citation2,7–10) With two exceptions, plant GH18 chitinases have a catalytic domain but no chitin-binding domain.Citation11,12) Class I chitinases have an N-terminal chitin-binding domain, whereas class II chitinases have only a catalytic domain homologous to that of class I. Class IV shares homology with class I but lacks several loops.Citation13) Class II-L chitinases are variants of class II enzymes and also lack several loops. Class III, IIIb, and V chitinases share a DxDxE motif around the catalytic cleft and a (β/α)8 barrel structure, but share very little homology (10–15%). Class III is a major GH18 class; these chitinases have three conserved disulfide bonds. Class IIIb is relatively minor, has no disulfide bonds, and has a pattern of chitin oligosaccharide degradation distinct from that of class III. Class V chitinases have a long insertion in comparison with classes III and IIIb and a distinct chitin degradation pattern.Citation14,15)
From several experiments using various types of chitinases derived from various plants, Taira et al.Citation10) showed that a chitinase with high antifungal activity contains both a catalytic domain of GH19 and a chitin-binding domain. Chitin oligosaccharides have function as elicitors and induce a defense response.Citation16) Therefore, we assume that some plant chitinases hydrolyze fungal cell wall chitin; although their activity does not directly inhibit infection with pathogenic fungi, they may be indirectly involved in the defense response.
Recently, a chitin elicitor receptor kinase 1 (CERK1) was found in Arabidopsis thaliana; CERK1 has a chitin-binding extracellular domain (LysM domain).Citation17) Nodulation (Nod) factor, a symbiotic signal between legumes and Rhizobium species, is a derivative of chitin oligosaccharides; the extracellular domain of its receptor is also a LysM domain.Citation18,19) Therefore, plant chitinases and chitin-binding proteins are of interest as important molecules involved in the interaction between plants and microorganisms.
In this study, we investigated the functions of chitinases in the moss Physcomitrella patens. The genome of this moss has been publishedCitation20) and gene targeting technology based on a high homologous recombination rate in this species has been established.Citation21) We identified 10 candidate chitinase genes on the basis of the presence of catalytic residues and overall sequence similarity with proteins annotated as chitinases. We also identified two candidate genes for CERKs by their sequence similarity to CERK1 from A. thaliana. The small number of candidate chitinase genes and their low structural similarity to each other make P. patens an attractive model for expression and functional analysis of plant chitinases. Using reverse transcription–quantitative real-time PCR (RT-qPCR), we analyzed the transcription of these genes after plant treatment with chitin oligosaccharides. We also succeeded in producing three recombinant chitinases and examined their enzymatic characteristics. On the basis of these data, we discuss the putative role of each chitinase in the defense response.
Materials and methods
Biological materials
Physcomitrella patens ssp. patens Gransden 2004 strainCitation20) was provided by Prof. Hasebe’s laboratory (National Institute for Basic Biology). It was cultured on BCDATG agar medium according to Nishiyama et al.Citation22) under the 16 h light/8 h dark cycles at 25 °C. Chitin and chitin beads were purchased from Seikagaku Kogyo Co. (Tokyo, Japan); chitin beads were from New England BioLabs (Ipswich, MA, USA). Escherichia coli BL21 (DE3) cells and the expression vector pET-22b were purchased from Novagen (Madison, WI, USA). All other reagents were of analytical grade.
Identification of candidate P. patens genes for chitinases and CERKs
BLAST searches were performed at the NCBI database against DNA and amino acid sequences with default parameters (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Signal peptides were identified by using the SignalP program (http://www.cbs.dtu.dk/services/SignalP/).Citation23) ClustalW (http://www.genome.jp/tools/clustalw/) was used for multiple alignments and phylogenetic analyses. The 10 identified chitinases were termed PpChi-Ia, PpChi-Ib, PpChi-Ic, PpChi-IIa, PpChi-IIb, PpChi-IIc, PpChi-II-L, PpChi-IV, PpChi-Va, and PpChi-Vb, and the two CERKs were termed PpCERK-a and PpCERK-b.
Chitin oligosaccharide treatment
Plants at the leafy stage (20 d after passage) were soaked in 100 μM solutions of chitin oligosaccharides [(GlcNAc)6, (GlcNAc)4, or (GlcNAc2)] in BCDATG liquid medium for 1, 3, or 6 h. Total RNA was extracted and used for gene expression analysis.
Reverse transcription–quantitative real-time PCR
Total RNA was isolated by using an RNeasy kit (Qiagen, Hilden, Germany) and treated with DNase I (Takara Bio, Kyoto, Japan) according to the manufacturers’ instructions. First-strand cDNA was synthesized on total RNA (300 ng) by using a SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) with a random hexamer primer. qPCR was carried out for target and reference genes on a 7300 Real-Time PCR System with Power SYBR Green PCR Master Mix (both from Applied Biosystems, Foster City, CA) under the following conditions: initial denaturation at 95 °C for 10 min and 40 cycles of denaturation at 95 °C for 15 s and annealing and extension at 60 °C for 1 min. The primer sets used for qPCR are listed in Table S1. Standard curves for absolute quantification were obtained from serially diluted PCR products obtained on genomic DNA as a template. Genomic DNA was isolated from P. patens protonemata by using an innuPREP Plant DNA Kit (Analytik Jena, Jena, Germany) and primer sets used for standard curves of each gene are listed in Table S2.
Plasmid construction and preparation of cell free extract of recombinant E. coli
To produce recombinant mature PpChi-Ia (Gln20-Ala301), PpChi-IV (Gln33-Cys288), and PpChi-Vb (Ala31-Asn369) in E. coli, full-length cDNA of each chitinase was cloned into pGEM-T. The open reading frame (ORF) without the putative signal sequence was amplified by PCR on each cDNA as a template. Primer sets are listed in Table S3. The amplified DNA fragments were digested with NdeI and BamHI and then ligated into pET-22b digested with the same enzymes. The resulting plasmids (pET-PpChi-Ia, pET-PpChi-IV, and pET-PpChi-Vb) were introduced into E. coli BL21 (DE3). E. coli cultures harboring recombinant plasmids were grown to the optical density at 600 nm of 0.6, isopropyl-β-d-thiogalactopyranoside was added (1 mM final concentration), and cultures were incubated for 24 h at 18 °C. The cells were harvested by centrifugation, suspended in 20 mM Tris–HCl buffer (pH 8.0), and disrupted by sonication. Cell debris was removed by centrifugation (10,000 × g, 10 min) and the supernatant was dialyzed against 10 mM sodium acetate buffer (pH 5.0) overnight. The insoluble material was removed by centrifugation at 10,000 × g for 20 min and used as the cell extract for protein purification.
Purification of recombinant PpChi-Ia
The cell extract was applied to an SP Sepharose Fast Flow column (GE Healthcare, Piscataway, NJ, USA) (10 mm × 50 mm) equilibrated with the dialysis buffer. The column was washed with the same buffer, and adsorbed proteins were eluted with 0.5 mM NaCl in the same buffer. The fractions containing chitinase activity were dialyzed against 20 mM Tris–HCl buffer (pH 8.0) and applied to a HiTrap Q HP column (GE) equilibrated with the dialysis buffer. The column was washed with the same buffer, and adsorbed proteins were eluted with a linear gradient of NaCl from 0 to 0.5 M in the same buffer. Active fractions were dialyzed against 10 mM sodium acetate buffer (pH 5.0) and applied to a HiTrap SP HP column (GE) equilibrated with the same buffer. Adsorbed proteins were eluted with a linear gradient of NaCl from 0 to 0.5 M in the same buffer. The active fraction was collected as PpChi-Ia.
Purification of PpChi-IV
The cell extract was mixed with one-third volume of 4 M ammonium sulfate. The mixture was applied to a Phenyl Superose column (GE) (5 mm × 50 mm) equilibrated with 10 mM sodium acetate buffer (pH 5.0) containing 1 M ammonium sulfate. The column was washed with the same buffer containing 1 M ammonium sulfate, and adsorbed proteins were eluted with a linear gradient of ammonium sulfate from 1 to 0 M in the same buffer. Fractions containing chitinase activity were dialyzed against 10 mM sodium acetate buffer (pH 5.0) and applied to a Mono-Q column (GE) (5 mm × 50 mm) equilibrated with the same buffer. The adsorbed proteins were eluted with a linear gradient of NaCl from 0 to 0.2 M in the same buffer. The active fraction was collected as PpChi-IV.
Purification of PpChi-Vb
The cell extract was applied to a HiTrap Q HP (GE) column equilibrated with 10 mM sodium acetate buffer (pH 5.0). Adsorbed proteins were eluted with a linear gradient of NaCl from 0 to 0.3 M in the same buffer. The active fraction was collected as PpChi-Vb.
Measurement of protein concentrations
Protein concentrations were measured by using the bicinchoninic acid method with bovine serum albumin as the standard.Citation24)
Assay of chitinase activity
Chitinase activity of each chromatographic fraction was assayed colorimetrically using glycol chitin or chitin beads. A sample (10 μL) was added to 250 μL of 0.2% (w/v) glycol chitin or 1% (w/v) chitin beads in a 5 mM sodium acetate buffer (PpChi-Ia and –IV, pH 5.0; PpChi-Vb, pH 4.0). The reaction mixture was incubated at 37 °C for 15 min and its reducing power was measured using ferri-ferrocyanide reagent according to Imoto and Yagishita.Citation25) Chitinase activity was estimated by the difference in absorbance of the reaction mixture and blank control at 420 nm (ΔA420). One unit of activity was defined as the amount of enzyme releasing 1 μmol of GlcNAc per min at 37 °C.
Electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) was performed according to LaemmliCitation26) in 15% polyacrylamide gels. Separated proteins were stained with Coomassie Brilliant Blue R250. A molecular weight marker kit (Apro Science, Tokushima, Japan) was used.
HPLC analysis of products of (GlcNAc)n digested by chitinases
The products of (GlcNAc)n (n = 4–6) hydrolysis by chitinases were analyzed on a TSK-Gel Amide-80 HPLC column (0.46 × 25 cm; Tosoh, Tokyo, Japan) according to Koga et al.Citation27) The reaction mixture consisting of 20 pmol of chitinase and 10 nmol of (GlcNAc)n in 100 μL of 4 mM sodium acetate buffer was incubated for 1, 5, 10, 20, or 30 min at 25 °C and immediately cooled in an ice bath. An aliquot (5 μL) was applied to the column. The reaction products were eluted with 70% (v/v) acetonitrile at a flow rate of 0.7 mL min−1 and absorbance was measured at 220 nm.
Antifungal assay
An antifungal assay was performed according to Schlumbaum et al.Citation6) with a modification. The fungus Trichoderma viride was cultured on potato dextrose broth with 1.5% (w/v) agar (PDA). An agar disk (6 mm in diameter) containing actively growing fungus was placed in the center of a Petri dish containing PDA. The dishes were incubated at room temperature for 12 h. Wells were punched in the agar at a distance of 15 mm from the center of the Petri dish and samples dissolved in 10 μL sterile distilled water were placed into the wells. The plates were incubated for 24 h at room temperature and then photographed.
Results
Candidate P. patens genes for chitinases and CERKs
Using BLAST searches, we found 10 candidate genes encoding chitinases with preserved active sites (Table ). This number was smaller than those of higher plants (24 genes in A. thaliana and 37 genes in Oryza sativa)Citation28), indicating that P. patens could be a convenient model to study chitinases. We also identified two CERK candidates (Table ); they had three LysM domains at the N-terminal region and a tyrosine kinase domain at the C-terminal region, similar to CERKs of higher plants.
Table 1. Chitinase and CERK candidate genes in P. patens.
Cloning and sequence analysis of PpChi cDNAs
Transcripts for six of the ten candidates were detected (Table ). We cloned six corresponding full-length cDNAs and compared their sequences with those of the predicted mRNAs and genomic DNAs in the NCBI database. Predicted PpChi-Ia had different splicing sites from the cloned cDNA (Fig. S1) and lacked two glutamic acid residues essential for chitin-degrading activity, which were found in the cloned cDNA, suggesting that the latter is the correct PpChi-Ia sequence.
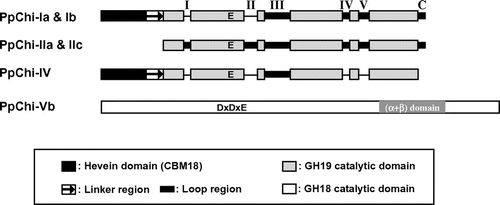
Multiple alignments of PpChi-I, -II, and -IV with other plant GH19 chitinases (Fig. S2) indicated that PpChi-IIa and -IIc have all the loop regions in the catalytic domains that are conserved in many typical class I and II chitinases in seed plants. PpChi-Ia and -IIb lacked loop I and II regions, although other loops and the chitin-binding domain were present. PpChi-IV lacked loops I, II, IV, and V and the C-terminal loop.
On the basis of alignments (Figs. S2 and S3) and phylogenetic analyses of the predicted amino acid sequences (Figs. S4 and S5), the 10 chitinases were divided into class I (PpChi-Ia, PpChi-Ib, PpChi-Ic), class II (PpChi-IIa, PpChi-IIb, PpChi-IIc), class II-L (PpChi-II-L), class IV (PpChi-IV), and class V (PpChi-Va, PpChi-Vb) (Fig. , Table ). The lengths of ORFs, predicted signal peptides, and mature proteins of six cloned cDNAs are shown in Table and the primary structure of the chitinases of P. patens were shown in Fig. .
Expression of chitinase and CERK genes in response to chitin oligosaccharides
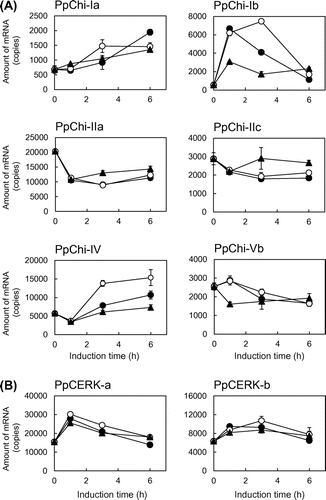
Genes encoding six PpChis (Ia, -Ib, -IIa, -IIc, IV, and -Vb) and both CERKs were expressed at considerable levels (Fig. , Table ). The expression profiles induced by chitin oligosaccharide treatments could be roughly categorized into three groups: 1) up-regulation (classes I and IV); 2) down-regulation (class II); and 3) no considerable changes (class V). However, different patterns observed within each group, e.g. PpChi-Ia and PpChi-Ib genes, and different responses to different oligosaccharides, e.g. PpChi-Ib gene, were observed. Both CERK genes were transiently up-regulated by chitin oligosaccharides after treatment. The expression level of each chitinase gene varied with the degree of polymerization of chitin oligosaccharides (Fig. (A)). In the case of PpChi-IV gene, the expression level with tetramer treatment was clearly higher than those with hexamer. The expression of PpChi-Vb gene had no considerable changes with tetramer and hexamer treatment, whereas the expression was down-regulated by dimer treatment. The expression of the CERK genes was less affected by the degree of polymerization (Fig. (B)).
Fig. 2. Expression analysis of chitinase genes by RT-qPCR.
Characterization of recombinant PpChi proteins
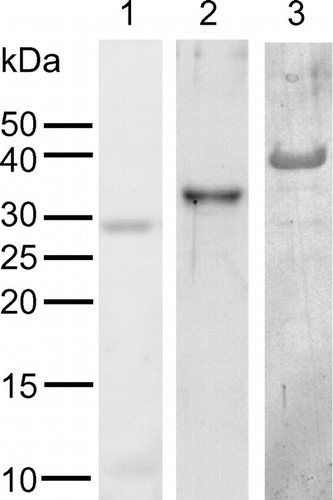
Heterologous expression and purification of recombinant proteins were successful for PpChi-Ia, PpChi-IV, and PpChi-Vb. On the other hands, recombinant PpChi-Ib, PpChi-IIa, and PpChi-IIc were found in inclusion bodies. The molecular masses of the recombinant proteins estimated by SDS–PAGE were 29 kDa for PpChi-Ia, 34 kDa for PpChi-IV, and 38 kDa for PpChi-Vb (Fig. ). The molecular mass of the recombinant PpChi-IV was predicted to be 27789 Da based on the sequence of the cloned cDNA. We do not have reliable explanation about this deference in SDS–PAGE. Because the fraction prepared from E. coli carrying pET-PpChi-IV showed chitinase activity and the specific band was detected on the gel, we used the fraction as a purified recombinant protein of PpChi-IV for further investigation.
Fig. 3. SDS–PAGE of recombinant PpChis.
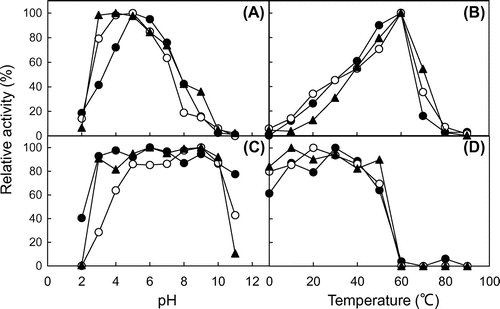
For all three chitinases, the optimum pH was 4.0–5.0 (Fig. (A)) and the optimum temperature was 60 °C (Fig. (B)). PpChi-Ia and Vb were stable between pH 3.0 and 10.0, and PpChi-IV was stable between pH 5.0 and 10.0 (Fig. (C)). All three chitinases were stable at temperatures up to 50 °C (Fig. (D)).
Fig. 4. Effect of pH and temperature on the chitinase activity.
Oligosaccharide degradation by PpChis
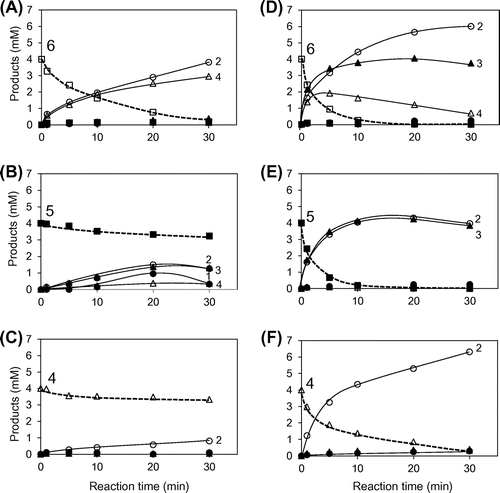
The degradation of (GlcNAc)4–6 catalyzed by PpChi-Ia and IV is shown in Fig. . In the reaction catalyzed by PpChi-Ia, (GlcNAc)6 was degraded into (GlcNAc)4 + (GlcNAc)2; (GlcNAc)5 into (GlcNAc)3 + (GlcNAc)2 or (GlcNAc)4 + GlcNAc; and (GlcNAc)4 into (GlcNAc)2 + (GlcNAc)2. Chitinase activity of PpChi-Ia decreased with the decreasing degree of polymerization of the substrate (Fig. (A)–(C)). In the reaction catalyzed by PpChi-IV, (GlcNAc)6 was degraded into (GlcNAc)4 + (GlcNAc)2 or (GlcNAc)3 + (GlcNAc)3; the produced tetramer was further degraded into (GlcNAc)2 + (GlcNAc)2. PpChi-IV more actively degraded low-molecular-weight substrates than PpChi-Ia did (Fig. (D)–(F)).
Fig. 5. Time-courses of the PpChi-Ia and -IV catalyzed reaction toward (GlcNAc)6–4. Time-course of the reaction products of (GlcNAc)6–4 by PpChi-Ia (A, B, and C) and PpChi-IV (D, E, and F).
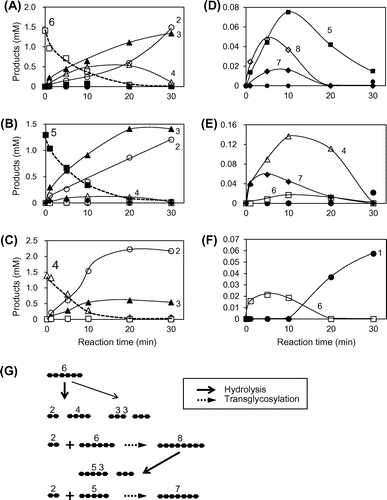
The reactions catalyzed by PpChi-Vb are shown in Fig. ; (GlcNAc)6 was degraded into (GlcNAc)4 + (GlcNAc)2 or (GlcNAc)3 + (GlcNAc)3 (Fig. (A)). However, (GlcNAc)5 was also produced; this reaction was not accompanied by GlcNAc formation, and (GlcNAc)8 and (GlcNAc)7, that is, products with a higher degree of polymerization than that of the substrate, were also generated (Fig. (D)). Similarly, (GlcNAc)5 was degraded into (GlcNAc)3 + (GlcNAc)2, and (GlcNAc)4 was produced without GlcNAc formation (Fig. (B)) but with the generation of (GlcNAc)7 (Fig. (E)). Similar results were obtained for (GlcNAc)4 (Fig. (C) and (F)). These results suggest that PpChi-Vb has transglycosylation activity in parallel with its hydrolase activity. The reaction model for (GlcNAc)6 is shown in Fig. (G). (GlcNAc)6 is first hydrolyzed into (GlcNAc)3 + (GlcNAc)3 or (GlcNAc)4 and (GlcNAc)2, and (GlcNAc)2 is released from the enzyme. (GlcNAc)6 is then combined with (GlcNAc)2 by transglycosylation, resulting in (GlcNAc)8, which is immediately hydrolyzed into (GlcNAc)5 + (GlcNAc)3. Similarly, (GlcNAc)7 is generated by transglycosylation reaction between (GlcNAc)5 derived from (GlcNAc)8 hydrolysate and (GlcNAc)2 from (GlcNAc)6 (Fig. (G)).
Fig. 6. Time courses of (GlcNAc)6–4 hydrolysis and transglycosylation catalyzed by PpChi-Vb.
Antifungal activity of PpChis
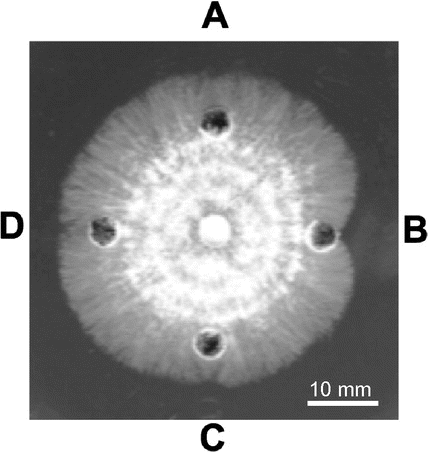
Antifungal activity of PpChi-Ia, PpChi-IV, and PpChi-Vb was examined using the hyphal extension inhibition assay on agar plates with T. viride as the test fungus. PpChi-Ia inhibited hyphal extension, whereas PpChi-IV and PpChi-Vb did not (Fig. ).
Discussion
In this study, we detected 10 putative PpChi genes (classes I, II, II-L, IV, and V) and two CERK genes in the genome of the moss P. patens, demonstrated the expression of six of the chitinase genes and both CERK genes, and characterized the activity of recombinant PpChis Ia, IV, and Vb. Our data suggest that P. patens chitinases function in the biological defense response and that chitinases of different classes play different roles.
We showed that, among the three recombinant PpChis analyzed, only PpChi-Ia inhibited hyphal extension of T. viride (Fig. ). Plant chitinases with antifungal activity have been reported in barley, tobacco, rye, and rice.Citation29–32) A small amount (2 μM) of rye seed chitinase-c (RSC-c) inhibited hyphal extension sufficiently, whereas a large amount (100 μM) of Bryum coronatum chitinase-A (BcChi-A) did not exhibit any antifungal activity.Citation13) PpChi-Ia inhibited hyphal extension at 300 pmol indicating that PpChi-Ia has a moderate antifungal activity. Suarez et al.Citation33) reported that the tobacco basic class I chitinase CHN A and its mutant that lacks the chitin-binding domain inhibited the growth of T. viride; CHN A was approximately five times more effective than the mutant. Taira et al.Citation31) showed in rye that a class I chitinase was a stronger inhibitor of hyphal elongation than a class II chitinase. These reports indicate that the chitin-binding domain of class I chitinases contribute to antifungal activity. These findings and the antifungal activity of PpChi-Ia detected in our study suggest that class I chitinases of P. patens may directly prevent plant invasion by fungal pathogens. The expression of PpChi-Ia and -Ib genes was up-regulated by chitin oligosaccharide elicitors with different expression profiles (Fig. (A)). This suggests that these two chitinases have antifungal role, although the enzymatic activity of PpChi-Ib has not been investigated yet.
Yamada et al.Citation34) showed that, in suspension-cultured rice cells, chitin dimers and trimers are less effective as elicitors than oligomers longer than a tetramer. We also found a stronger response of several PpChi genes to chitin tetramer and hexamer than to dimer (Fig. (A)). In suspension-cultured rice cells, GlcNAc monomer had no activity as an elicitor as well as control experiment [without (GlcNAc)n].Citation34) Although we have not done these experiments, it is well predicted that chitinase and CERK genes in the moss plant also do not respond to GlcNAc monomer and culture solution without (GlcNAc)n. PpChi-Ia more efficiently degraded the hexamer (and produced the tetramer and dimer) than the penta- and tetramer, whereas PpChi-IV also efficiently degraded the pentamer and tetramer (Fig. ).
In GH19 chitinases, loop regions strongly affect the degradation of chitin oligosaccharides. Taira et al.Citation13) have reported that activity of RSC-c, which has all loop regions, against chitin oligosaccharide hexamer is about 100 times that against the tetramer. Ohnuma et al.Citation35,36) reported the crystal structures of two RSC-c–(GlcNAc)4 complexes; in one complex, (GlcNAc)4 was bound to subsites +1,+2,+3, and +4, and the other complex contained two (GlcNAc)4 molecules, one bound to the same subsites and the other one to subsites −4, −3, −2, and −1. The substrate-binding cleft of RSC-c is longer than that of “loopless” class IV chitinase because of loop location at the end of the cleft. The extended substrate binding cleft hampers (GlcNAc)4 binding in the active center.
The chitinase BcChi-A lacks loops I, II, IV, and V and C-terminal loop. The chitin-degrading activity of BcChi-A is higher than that of RSC-c: 10 times for (GlcNAc)6, 100 times for (GlcNAc)5, and 1000 times for (GlcNAc)4. The crystal structure of BcChi-A in a complex with (GlcNAc)4 showed that its substrate-binding cleft is composed of subsites −2, −1,+1, and +2.Citation37) This result implies that the structure of BcChi-A is suitable for degrading the tetramer and that “loopless” GH19 chitinases such as BcChi-A are optimized for shorter substrates.
The structure of PpChi-IIa and -IIc are similar to that of RSC-c, suggesting that PpChi-II and RSC-c have similar properties. PpChi-Ia, which lacks two loop regions, poorly degraded the tetramer. We expect that PpChi-IIa and -IIc, which have all loops, would have very low activity against the tetramer. PpChi-IIa and -IIc were constitutively expressed and were down-regulated by chitin oligosaccharide tetramer and hexamer. In mosses, PpChi-IIa and -IIc might release chitin elicitors (tetramer and higher) from cell-wall chitin of fungal pathogens, and thus play a role in the defense system.
Activity of PpChi-IV against chitin tetramer was higher than that of PpChi-Ia (Fig. ). The response of PpChi-IV gene expression to chitin oligosaccharide treatment was slower than those of other chitinase genes, and the expression level continued to increase by 6 h (Fig. (A)). In the presence of the tetramer, the expression level of PpChi-IV was higher than with the hexamer. As the amounts of chitin elicitors with the degree of polymerization higher than hexamer could be large in the early stage of fungal invasion, and the amounts of tetramer could increase in the late stage, the class IV chitinase might work in the late stage of the defense response in P. patens.
Reports on plant GH18 chitinases with antifungal activity are limited. The Nicotiana tabacum chitinase NtChi-V has relatively strong antifungal activity,Citation38) whereas the anti-fungal activity of the A. thaliana chitinase AtChi-C (class V) is extremely low, and the Cycas revoluta chitinase CrChi-A (class V) does not have any antifungal activity.Citation14,38) A class V chitinase in the legume Medicago truncatula (renamed as Nod factor hydrolase) degrades Nod factor but not chitin oligosaccharides.Citation39) Therefore, legume class V chitinases might be involved in symbiosis rather than biological defense.
Although transglycosylation activity is observed in GH18 chitinases of many bacteria, CrChi-A is the first example of a plant chitinase with such activity,Citation14) and PpChi-Vb described here is the second example. As there are no reports of transglycosylation activity of class V chitinases from angiosperms, the transglycosylation function of angiosperm chitinases might have been lost in the course of evolution. Transglycosylation activity of class V chitinases may enhance elicitor activity by increasing the degree of polymerization of chitin oligosaccharides. Alternatively, the function of PpChi-Vb might not be related to biological defense because its expression did not respond to chitin oligosaccharides.
We speculate that, in P. patens, when fungal hyphae invade plant intercellular spaces, constitutively expressed class II chitinases release chitin oligomers (tetramer and higher) from fungal cell walls. The oligomers are then recognized by CERKs and trigger the expression of antifungal class I chitinases, which directly prevent fungal invasion by degrading cell wall chitin. The class IV chitinase is expressed at the late stages of fungal invasion and degrades chitin elicitors, and then the defense response is completed.
Salzer et al. showed a role of chitinases in symbiosis between Picea abies and mycorrhiza.Citation40) Chitinases of the host plant cleave chitinous elicitors (tetramer and higher) from the mycorrhizal fungus to monomers and dimers, and thus prevent host defense. According to the authors, at the early stage, only some fungal chitin elicitors reach their receptors at the host plasma membrane and initiate the hypersensitive response. The remaining fungal elicitors are degraded by host chitinases, which reduces plant defense reactions and allows symbiotic interactions. These observations suggest that plant chitinases play various roles in plant–microbe interactions. To clarify their roles, functional analysis is essential; P. patens will be a very useful model for further investigation of plant chitinases because the number of chitinases is smaller than that of higher plantsCitation28) and gene targeting technology has been established.Citation21)
Author contributions
SK and TT conceived and designed research. SK and RT performed experiments. TT and TU conducted experiments and contributed to data elaboration. SK, TT, and TU wrote the manuscript. All authors read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1224640.
TBBB_1224640_Supplementary_Files.pdf
Download PDF (633.7 KB)Acknowledgments
The authors greatly appreciate the gift of spores of P. patens from Prof. Mitsuyasu Hasebe, National Institute for Basic Biology. The authors also thank Yoko Mahoe and Yoshinobu Kina, University of the Ryukyus, for technical assistance.
References
- Kasprzewska A. Plant chitinases-regulation and function. Cell. Mol. Biol. Lett. 2003;8:809–824.
- Collinge DB, Kragh KM, Mikkelsen JD, et al. Plant chitinases. Plant J. 1993;3:31–40.10.1046/j.1365-313X.1993.t01-1-00999.x
- Graham LS, Sticklen MB. Plant chitinases. Can. J. Bot. 1994;72:1057–1083.10.1139/b94-132
- Selitrennikoff CP. Antifungal proteins. Appl. Environ. Microbiol. 2001;67:2883–2894.10.1128/AEM.67.7.2883-2894.2001
- Theis T, Stahl U. Antifungal proteins: targets, mechanisms and prospective applications. Cell Mol. Life Sci. 2004;61:437–455.10.1007/s00018-003-3231-4
- Schlumbaum A, Mauch F, Vögeli U, et al. Plant chitinases are potent inhibitors of fungal growth. Nature. 1986;324:365–367.10.1038/324365a0
- Shinshi H, Neuhaus J-M, Ryals J, et al. Structure of a tobacco endochitinase gene: evidence that different chitinase genes can arise by transposition of sequences encoding a cysteine-rich domain. Plant Mol. Biol. 1990;14:357–368.10.1007/BF00028772
- Melchers L, Groot M. A new class of tobacco chitinases homologous to bacterial exo-chitinases displays antifungal activity. Plant J. 1994;5:469–480.10.1046/j.1365-313X.1994.5040469.x
- Yamagami T, Mine Y, Ishiguro M. Complete amino acid sequence of chitinase-a from bulbs of gladiolus (Gladiolus gandavensis). Biosci. Biotechnol. Biochem. 1998;62:386–389.10.1271/bbb.62.386
- Taira T. Structures and antifungal activity of plant chitinases. J. Appl. Glycosci. 2010;57:167–176.10.5458/jag.57.167
- Onaga S, Taira T. A new type of plant chitinase containing LysM domains from a fern (Pteris ryukyuensis): roles of LysM domains in chitin binding and antifungal activity. Glycobiology. 2008;18:414–423.10.1093/glycob/cwn018
- Inamine S, Onaga S, Ohnuma T, et al. Purification, cDNA cloning, and characterization of LysM-containing plant chitinase from horsetail (Equisetum arvense). Biosci. Biotechnol. Biochem. 2015;79:1296–1304.10.1080/09168451.2015.1025693
- Taira T, Mahoe Y, Kawamoto N, et al. Cloning and characterization of a small family 19 chitinase from moss (Bryum coronatum). Glycobiology. 2011;21:644–654.10.1093/glycob/cwq212
- Taira T, Hayashi H, Tajiri Y, et al. A plant class V chitinase from a cycad (Cycas revoluta): biochemical characterization, cDNA isolation, and posttranslational modification. Glycobiology. 2009;19:1452–1461.10.1093/glycob/cwp119
- Ohnuma T, Numata T, Osawa T, et al. A class V chitinase from Arabidopsis thaliana: gene responses, enzymatic properties, and crystallographic analysis. Planta. 2011;234:123–137.10.1007/s00425-011-1390-3
- Shibuya N, Minami E. Oligosaccharide signalling for defence responses in plant. Physiol. Mol. Plant Pathol. 2001;59:223–233.10.1006/pmpp.2001.0364
- Miya A, Albert P, Shinya T, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2007;104:19613–19618.10.1073/pnas.0705147104
- Limpens E, Franken C, Smit P, et al. LysM domain receptor kinases regulating rhizobial nod factor-induced infection. Science. 2003;302:630–633.10.1126/science.1090074
- Radutoiu S, Madsen LH, Madsen EB, et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature. 2003;425:585–592.10.1038/nature02039
- Rensing SA, Lang D, Zimmer AD, et al. The physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–69.10.1126/science.1150646
- Schaefer DG, Zrÿd JP. Efficient gene targeting in the moss Physcomitrella patens. Plant J. 1997;11:1195–1206.10.1046/j.1365-313X.1997.11061195.x
- Nishiyama T, Hiwatashi Y, Sakakibara K, et al. Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res. 2000;7:9–17.10.1093/dnares/7.1.9
- Bendtsen JD, Nielsen H, von Heijne G, et al. Improved prediction of signal peptides: Signalp 3.0. J. Mol. Biol. 2004;340:783–795.10.1016/j.jmb.2004.05.028
- Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85.10.1016/0003-2697(85)90442-7
- Imoto T, Yagishita K. A simple activity measurement of lysozyme. Agric. Biol. Chem. 1971;35:1154–1156.10.1080/00021369.1971.10860050
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.10.1038/227680a0
- Koga D, Yoshioka T, Arakane Y. HPLC analysis of anomeric formation and cleavage pattern by chitinolytic enzyme. Biosci. Biotechnol. Biochem. 1998;62:1643–1646.10.1271/bbb.62.1643
- Fenghua Xu, Fan Chengming, He Yueqiu. Chitinases in Oryza sativa ssp. japonica and Arabidopsis thaliana. J. Genet Genomics. 2007;34:138–150.
- Jacobsen S, Mikkelsen JD, Hejgaard J. Characterization of two antifungal endochitinases from barley grain. Physiol. Plant. 1990;79:554–562.10.1111/ppl.1990.79.issue-3
- Iseli B, Boller T, Neuhaus JM. The N-terminal cysteine-rich domain of tobacco class I chitinase is essential for chitin binding but not for catalytic or antifungal activity. Plant. Physiol. 1993;103:221–226.10.1104/pp.103.1.221
- Taira T, Yamagami T, Aso Y, et al. Localization, accumulation, and antifungal activity of chitinases in rye (Secale cereale) seed. Biosci. Biotechnol. Biochem. 2001;65:2710–2718.10.1271/bbb.65.2710
- Truong NH, Park SM, Nishizawa Y, et al. Structure, heterologous expression, and properties of rice (Oryza sativa L.) family 19 chitinases. Biosci. Biotechnol. Biochem. 2003;67:1063–1070.10.1271/bbb.67.1063
- Suarez V, Staehelin C, Arango R, et al. Substrate specificity and antifungal activity of recombinant tobacco class I chitinases. Plant Mol. Biol. 2001;45:609–618.10.1023/A:1010619421524
- Yamada A, Shibuya N, Kodama O, et al. Induction of phytoalexin formation in suspension-cultured rice cells by N-acetylchito oligo saccharides. Biosci. Biotechnol. Biochem. 1993;57:405–409.10.1271/bbb.57.405
- Ohnuma T, Numata T, Osawa T, et al. Crystal structure and chitin oligosaccharide-binding mode of a ‘loopful’ family GH19 chitinase from rye, Secale cereale, seeds. FEBS J. 2012;279:3639–3651.10.1111/j.1742-4658.2012.08723.x
- Ohnuma T, Umemoto N, Kondo K, et al. Complete subsite mapping of a “loopful” GH19 chitinase from rye seeds based on its crystal structure. FEBS Lett. 2013;587:2691–2697.10.1016/j.febslet.2013.07.008
- Ohnuma T, Umemoto N, Nagata T, et al. Crystal structure of a “loopless” GH19 chitinase in complex with chitin tetrasaccharide spanning the catalytic center. Biochim. Biophys. Acta. 2014;1844:793–802.10.1016/j.bbapap.2014.02.013
- Ohnuma T, Taira T, Fukamizo T. Antifungal activity of recombinant class V chitinases from Nicotiana tabacum and Arabidopsis thaliana. J. Appl. Glycosci. 2012;59:47–50.10.5458/jag.jag.JAG-2011_019
- Tian Y, Liu W, Cai J, et al. The nodulation factor hydrolase of Medicago truncatula: characterization of an enzyme specifically cleaving rhizobial nodulation signals. Plant Physiol. 2013;163:1179–1190.10.1104/pp.113.223966
- Salzer P, Hebe G, Hager A. Cleavage of chitinous elicitors from the ectomycorrhizal fungus Hebeloma crustuliniforme by host chitinases prevents induction of K+ and Cl− release, extracellular alkalinization and H2O2 synthesis of Picea abies cells. Planta. 1997;203:470–479.10.1007/s004250050216