Abstract
Canavalin is a vicilin-class (7S) storage protein found in sword bean (Canavalia gladiata). Our previous report indicated that canavalin is precipitated by the addition of 20 mM MgCl2 to crude sword bean extract. Here, we examined the solubility changes induced by the addition of Mg2+ and Ca2+ at various concentrations. Canavalin tended to be insolubilized at relatively low concentrations of MgCl2 (< 20 mM) and solubilized at relatively high concentrations (> 20 mM). In addition, canavalin was slightly insolubilized in the presence of NaCl. Overall, the results revealed that solubility changes are reversible and depend on the concentration of divalent cations. Therefore, we suggested a reaction scheme that describes the effects of divalent cations on the solubility of canavalin, which would facilitate the study of its physiological function and the application of canavalin in the food processing industry.
Graphical abstract
The solubility changes of canavalin are reversible and depend on the concentration of divalent cations.
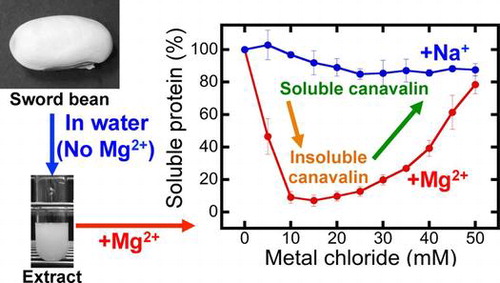
White sword bean (Canavalia gladiata) is a part of the traditional diet in the Asian tropics and subtropicsCitation1,2) and is relatively resistant to pests and most diseases.Citation3) Since the dried beans contain approximately 25% crude proteins,Citation1,4) they are good potential sources of protein. We have previously reported a method for the extraction of some proteins from sword bean using only distilled water.Citation5) In addition, the predominant protein in the crude extract was determined to be canavalin.Citation5)
Canavalin is the major storage protein of sword bean and jack bean (Canavalia ensiformis) with a molecular mass of 47.6 kDa, classified as 7S seed globulin or legume vicilin.Citation6) It was first isolated from jack bean,Citation7) and later its primary structure was determined in both bean species using cDNA sequence.Citation8–11) These two primary structures differ in only two out of 419 amino acids of canavalinCitation12,13) and have high homology with other legume 7S globulins,Citation12–15) which probably shows that they may also similar tertiary structure, physiological function, and physicochemical properties. Although the tertiary structure of sword bean canavalin has not been yet determined, several X-ray crystal structure analyses showed that jack bean canavalin is a homotrimer.Citation16–20) The tertiary structure highly resembles that of soybean β-conglycinin, a soybean 7S globulin.Citation12) Soybean 7S globulin is precipitated by the addition of divalent cations.Citation21) This characteristic of soybean 7S globulin is utilized for the processing of tofu. If canavalin were also to possess similar characteristics, this protein could be useful for the manufacture of processed foods.
In our previous study, we described a method for the extraction of proteins from sword bean in distilled water and their precipitation by the addition of 20 mM MgCl2 in the crude extract.Citation5) This was the first study that reported the precipitation of canavalin in the presence of MgCl2 and showed that a detailed investigation of canavalin precipitation might be important for the study of its physiological function and physicochemical properties.
In this study, we examined the effects of the addition of MgCl2, CaCl2, and NaCl to the crude sword bean extract at various concentrations on the solubility of canavalin. Our findings might provide useful information for the study of the physiological functions and physicochemical properties of canavalin and its application in food industry.
Materials and methods
Materials
White sword beans were purchased from Morika Beiten (Nara, Japan) and general chemical reagents from Wako Pure Chemical Industries (Osaka, Japan).
Preparation of sword bean extract
A sword bean extract was prepared as described previously.Citation5) Briefly, soaked sword beans were ground for 5 min in eight volumes (v/w) of distilled water using a hand blender (CSB-77JBSTRW; Cuisinart, Stamford, CT) on ice, and the suspension was sieved through a cotton cloth. The extract was centrifuged at 9,100 × g for 10 min at 4 °C, and the supernatant was used for analysis.
SDS–PAGE
Samples were mixed with 0.33 volumes of SDS (0.25 M Tris-HCl at pH 7.0, containing 4% SDS, 5% 2-mercaptoethanol, and 40% glycerol) and incubated in a water bath at 100 °C for 5 min. SDS–PAGE was conducted using sample volumes corresponding to 0.25 μL of the sword bean extract; this volume contained approximately 4.0 μg proteins in the absence of divalent cations.Citation5) SDS–PAGE was carried out on 10% polyacrylamide gels at a constant current of 12.5 mA for 2.5 h as described previously.Citation22) Proteins were stained with 0.25% Coomassie Brilliant Blue R-250. The molecular weight standard was purchased from Life Technologies (Tokyo, Japan).
Analysis of supernatant proteins
The sword bean extract was incubated at 25 °C for 5 min. Next, 0.11 volumes of MgCl2, CaCl2, NaCl, or distilled water (control) were added to the incubated sample at various concentrations, mixed, and incubated at 25 °C for 15 min and on ice for 5 min. The mixtures were centrifuged at 9,100 × g for 20 min at 4 °C. The supernatant was diluted 15-fold with distilled water. The supernatant proteins were analyzed by 10% SDS–PAGE.
Determination of residual canavalin ratio
The intensity of canavalin bands on SDS-polyacrylamide gel was quantified by ImageJ (National Institutes of Health, Bethesda, MD).Citation23 The residual canavalin ratio was expressed as the percentage of band intensity of the sample with added metal chloride to that of the sample with distilled water. Data were expressed as mean ± standard deviation of three independent experiments.
Analysis of insoluble canavalin solubilization
Insoluble canavalin was prepared by the addition of 15 mM MgCl2 or 10 mM CaCl2 to sword bean extract. Canavalin precipitated in the former was suspended in 60 mM MgCl2 or distilled water, whereas that precipitated in the latter was suspended in 200 mM CaCl2 or distilled water. The mixtures were centrifuged at 9,100 × g for 20 min at 4 °C. The supernatant was diluted 15-fold with distilled water to adjust the concentration of divalent cations to less than 15 mM. The precipitates were dissolved in a volume of 8 M urea equal to that of the initial extract. Proteins were analyzed using 10% SDS–PAGE.
Analysis of soluble canavalin insolubilization
Soluble canavalin was prepared by the addition of 60 mM MgCl2 or 200 mM CaCl2 to sword bean extract. The solutions were diluted 4- or 20-fold, respectively, with distilled water. The diluted mixtures were centrifuged at 9,100 × g for 20 min at 4 °C. The MgCl2 supernatant was diluted threefold with distilled water such that the MgCl2 concentration was 5 mM. The CaCl2 concentration of the CaCl2 supernatant is adjusted to 10 mM. The precipitates were prepared as described for the analysis of insoluble canavalin solubilization. Proteins were analyzed using 10% SDS–PAGE.
Curve fitting analysis
The residual canavalin ratio was plotted against each metal chloride concentration. The plots were fitted to the following equation using the KaleidaGraph 4.5 software (Synergy Software, PA, USA):
where r is the residual canavalin ratio at a metal chloride concentration C, rmax is the maximum ratio, rmin is the minimum ratio, Cm is the midpoint metal chloride concentration, and d is the slope coefficient at the midpoint.Citation24,25) Data represent the average ± standard deviation of five independent experiments.
Results
Effects of MgCl2 and NaCl on canavalin solubility
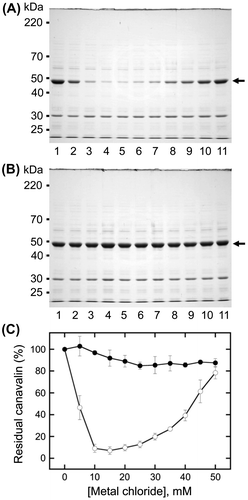
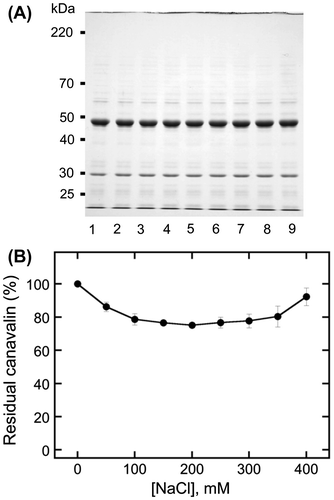
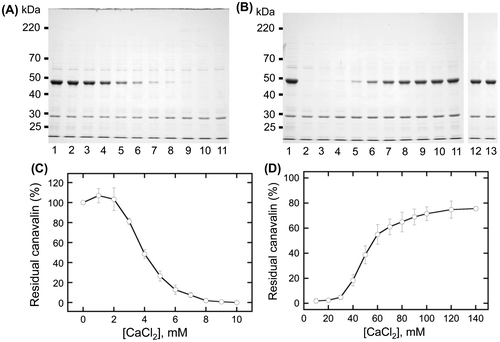
We first investigated the MgCl2 and NaCl concentration-dependency of canavalin precipitation (Fig. ). The results showed that the minimum ratio of residual canavalin was 7.1 ± 3.5% at 15 mM MgCl2, whereas the maximum was 78.4 ± 5.6% at 50 mM MgCl2. Therefore, canavalin was insolubilized at relatively low concentrations of MgCl2 (< 20 mM) and solubilized at relatively high concentrations (> 20 mM). When NaCl was added into the crude extract, canavalin solubilization was slightly decreased, and the minimum ratio of residual canavalin was 84.9 ± 3.3% at 25 mM NaCl. To investigate whether canavalin solubilization was highly decreased at NaCl concentrations of more than 50 mM, we also studied it at the range of 100–400 mM (Fig. ). The results showed that at 100–350 mM, approximately 75% of canavalin remained in the supernatant (Fig. (B)). The minimum ratio of residual canavalin was 75.1 ± 1.2% at 200 mM NaCl, whereas the maximum was 92.2 ± 5.3% at 400 mM NaCl. Therefore, although canavalin was soluble in the presence of Na+, Mg2+ had a more significant effect on canavalin solubility.
Fig. 1. Effects of magnesium chloride (MgCl2) and sodium chloride (NaCl) concentrations on canavalin solubility.
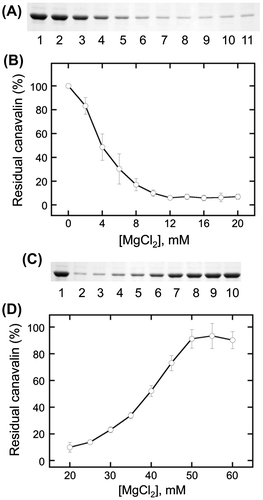
Changes in canavalin solubility induced by MgCl2
Canavalin reaction to MgCl2 was divided into the insolubilization phase in the range of 0–20 mM MgCl2 and the solubilization phase in the range of 20–50 mM MgCl2 (Fig. (C)). Therefore, the effect of MgCl2 on canavalin solubility was investigated separately in each phase (Fig. ). In the range of 0–20 mM MgCl2, soluble canavalin was highly decreased with the increasing concentration of MgCl2 (Fig. (A)). The reduction of canavalin in the supernatant was more than 90% (Fig. (B)) at concentrations higher than 12 mM MgCl2, reaching a maximum reduction at 16 mM MgCl2 (5.9 ± 2.3%). In the range of 20–60 mM, supernatant canavalin was sigmoidally increased with the increasing concentration of MgCl2, reaching a plateau at 50 mM MgCl2 (Fig. (C) and (D)). The highest amount of supernatant canavalin was 91.2 ± 7.0% at 55 mM MgCl2. These results showed that canavalin tends to be insolubilized at relatively low concentrations of MgCl2 (< 20 mM) and solubilized at relatively high concentrations (> 20 mM).
Fig. 2. Effects of high sodium chloride (NaCl) concentrations on canavalin solubility.
Effect of CaCl2 on canavalin solubility
To investigate the specificity of divalent cations, we also examined the effect of CaCl2 on the solubility of canavalin (Fig. ). Canavalin reaction to CaCl2 was divided into the insolubilization phase in the range of 0–10 mM CaCl2 (Fig. (A) and (C)) and the solubilization phase in the range of 20–60 mM CaCl2 (Fig. (B) and (D)). Canavalin completely disappeared in the range of 9–10 mM (Fig. (C)); it was sigmoidally increased by approximately 75% in the range of 10–140 mM (Fig. (D)); whereas 75.1 ± 1.2% was solubilized at 200 mM (Data not shown). These results indicated that CaCl2 played a similar role with that of MgCl2 and that the concentration of divalent cations significantly affects the solubility of canavalin.
Fig. 3. Analysis of canavalin solubility induced by magnesium chloride (MgCl2).
Comparison between effects of Mg2+ and Ca2+ on canavalin solubility
To compare the effect of Mg2+ and Ca2+ on canavalin solubility, the midpoint concentrations (Cm) in the insolubilization and solubilization phases were determined by curve fitting analysis (Figs. (B), (D) and (C), (D)). As summarized in Table , the Cm value of MgCl2 in the insolubilization phase was similar to that of CaCl2. However, MgCl2 induced the insolubilization of canavalin at 2 mM (Fig. (B)), whereas CaCl2 at the same concentration did not (Fig. (C)). The Cm value of MgCl2 in the solubilization phase was lower than that of CaCl2 (Table ). In addition, canavalin was almost completely recovered by the addition of MgCl2 (Fig. (D)), but only incompletely by the addition of CaCl2 (Fig. (D)). Overall, the results suggested that the effect of Mg2+ on the solubilization or insolubilization of canavalin was stronger than that of Ca2+.
Table 1. Midpoint concentrations for the insolbulization and solubilization of canavalin.
Reversibility of canavalin solubility
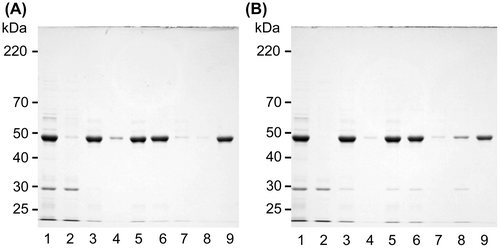
We examined whether insolubilized canavalin was solubilized in distilled water or in the presence of MgCl2 and CaCl2 (Fig. ). Canavalin was first insolubilized by the addition of 15 mM MgCl2 (Fig. (A), lanes 2 and 3), and most canavalin was observed in the precipitate fraction (Fig. (A), lane 3). The precipitated proteins were suspended in distilled water (Fig. (A), lanes 4 and 5), and only a small amount was dissolved. In contrast, the highest amount of precipitated proteins was dissolved in 60 mM MgCl2 (Fig. (A), lanes 6 and 7), and then, the insolubilization of resolubilized proteins was increased with decreasing concentrations of MgCl2 from 60 to 15 mM (Fig. (A), lanes 8 and 9).
Fig. 4. Effects of calcium chloride (CaCl2) concentration on canavalin solubility.
Canavalin was first insolubilized by the addition of 10 mM CaCl2 (Fig. (B), lanes 2 and 3). The precipitated proteins were suspended in distilled water (Fig. (B), lanes 4 and 5), and only a small amount was dissolved. The precipitated proteins were also suspended in 200 mM CaCl2 (Fig. (B), lanes 6 and 7), in which the highest amount was dissolved. The insolubilization of resolubilized proteins was increased with the decreasing concentration of CaCl2 from 200 to 10 mM (Fig. (B), lanes 8 and 9). These results indicated that CaCl2 also induces reversible solubility changes in canavalin.
It was also examined whether canavalin solubilized in the presence of Mg2+ and Ca2+ at relatively high concentrations was precipitated by decreasing the concentration of MgCl2 or CaCl2, respectively (Fig. ). Canavalin was solubilized by the addition of 60 mM MgCl2 (Fig. (A)) or 200 mM CaCl2 (Fig. (B)), and the soluble proteins were precipitated with the decreasing concentration of MgCl2 (Fig. (A), lane 5) or CaCl2 (Fig. (B), lane 5), respectively. The results indicated that canavalin was reversibly solubilized and insolubilized by the changing concentrations of Mg2+ and Ca2+.
Fig. 5. Solubility changes to insolubilized canavalin.
Discussion
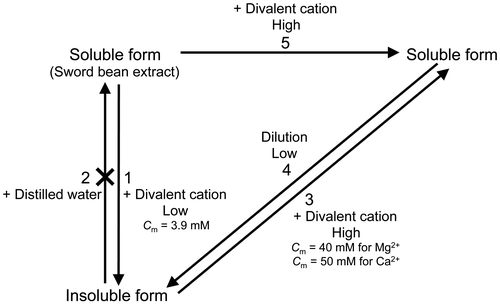
In our previous study, we indicated that canavalin is precipitated by the addition of 20 mM MgCl2,Citation5) whereas in the present study, we showed that canavalin was insolubilized in the presence of divalent cations at relatively low concentrations and solubilized in the presence of the same cations at relatively high concentrations. In addition, we formulated a schematic representation of the reversible induction of canavalin insolubilization and solubilization by changing concentrations of Mg2+ and Ca2+ (Fig. ). Soluble canavalin was prepared as described previouslyCitation5) and precipitated by the addition of MgCl2 or CaCl2 at relatively low concentrations, but not by the addition of NaCl (arrow 1). The insolubilized canavalin was not resolubilized in distilled water (arrow 2), but only in the presence of Mg2+ and Ca2+ at relatively high concentrations (arrow 3). In addition, the soluble canavalin was not precipitated by the addition of 60 mM Mg2+ or 200 mM Ca2+ (arrow 5), but it was with the decreasing concentration of Mg2+ and Ca2+ (arrow 4). Based on these results, we assumed that the soluble canavalin prepared by the addition of relatively high concentration of divalent cations to the crude extract was the same with that prepared by the addition of relatively high concentration of divalent cations to insoluble canavalin.
Fig. 6. Solubility changes to solubilized canavalin.
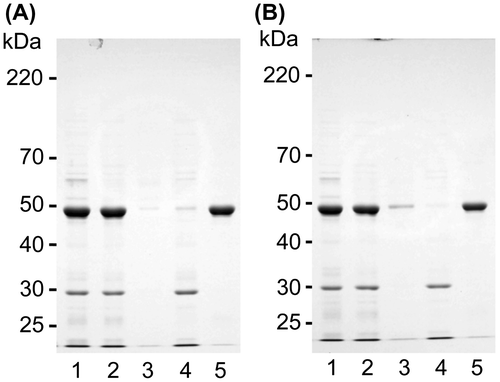
Fig. 7. Schematic representation of canavalin solubility.
In the solubilization phase, the Cm value was determined at 40 mM for Mg2+ and at 50 mM for Ca2+. The results clearly indicated that the insolubilized canavalin was more easily solubilized in a solution containing Mg2+ than in a solution containing Ca2+. In the insolubilization phase, the Cm value was determined at 3.86 mM for Mg2+ and 3.93 mM for Ca2+. The similar Cm values indicated that the effects of Mg2+ and Ca2+ on canavalin insolubilization do not differ significantly. However, the sigmoidal graphs of canavalin insolubilization (Figs. (B) and (C)) suggested that the protein shows a higher sensitivity to Mg2+ than to Ca2+ from the different initial concentrations for the insolubilization. Our previous study indicated that different metal ion species influence the Cm value of protein association during tofu formation.Citation24) The Cm value is significantly negatively correlated with the stability constant of EDTA for metal ions, and this strong correlation influences the interaction between metal ions and the carboxyl groups of soymilk proteins. Studies on the primary structure of canavalinCitation10,11) indicate that the 419 amino acid residues of the matured form of canavalin include 36 glutamic acid residues and 23 aspartic acid residues. Therefore, changes in canavalin solubility might be also induced by the interaction between metal ions and the carboxyl groups of the protein. However, it was difficult to clarify the mechanism underlying the modulation of solubility change, because the sword bean extract contained many non-proteinaceous substances, such as polysaccharides, that might also interact with canavalin. Thus, further studies are needed to reveal the underlying molecular mechanism.
Sword bean contains abundant calcium and magnesium ions.Citation26) Based on studies by Eknkaya et al.,Citation26) we estimated the Ca2+ and Mg2+ concentrations in our sword bean extract to be approximately 4.6 and 8.8 mM, respectively. The divalent cations inherently present in sword bean affect the primary solubility change of canavalin. Thus, our results must be interpreted with consideration of the fact that the Cm values in this study indicate the midpoint concentration for the insolubilization and solubilization in the sword bean extract prepared by the method we describe, but might not represent the essential features of canavalin. At present, since highly pure canavalin is insoluble in distilled water (Fig. ), it is experimentally difficult to determine the Cm value of purified canavalin for the divalent cations. However, it would be important to determine the Cm value of the unrefined canavalin in the extract to facilitate the use of canavalin in food processing. For example, tofu is also processed by the addition of divalent cations to extracts of soybean, which also contains abundant divalent cations. As we have previously reported,Citation5) the methodologies used in tofu production could be applied to the development of processed foods using canavalin. In our previous study, we determined Cm values for tofu formation, and a comparison of Cm values between various metal ions clarified the role of metal ions in tofu formation.Citation24) The Cm value for canavalin precipitation would thus be useful information for the development of processed foods using canavalin.
Some proteins are precipitated by the addition of salts, whereas others are solubilized. For example, during tofu formation, soybean proteins are precipitated by an increase in divalent cations,Citation24,27) while chymosin B, β-lactoglobulin B and pumpkin seed globulin demonstrate salting-in behavior—that is, they are solubilized in the presence of high salt concentrations.Citation28) In the present study, canavalin was insolubilized by the addition of divalent cations at relatively low concentrations, but was solubilized by the addition of the same cations at relatively high concentrations. Briefly, canavalin solubility can be modulated by increasing divalent cation concentration. The modulation of canavalin solubility is interesting from the viewpoint of protein chemistry. With increase in the divalent cation concentration, soluble canavalin decreased in the insolubilization phase, but increased in the solubilization phase. Therefore, canavalin insolubilization was altered to solubilization in a salt concentration-dependent manner. The phase transition is a unique property of canavalin. We assumed that the transition might be induced by the interaction between canavalin and non-proteinaceous substances or/and by some structural changes. However, the underlying molecular mechanism remains unclear, and further studies are needed.
The reversible change in solubility would be useful for the establishment of an inexpensive and simple method for the purification of canavalin. Studies on the primary structure of canavalinCitation10,11) indicated that the 419 amino acid residues of the matured form of canavalin include 52 leucine residues. A previous study describes that the consumption of leucine in combination with exercise is effective in the prevention and improvement of sarcopenia.Citation29) It is also reported that, since whey proteins have relatively high leucine content (approximately 9.5 g of leucine per 100 g of proteins), the intake of whey proteins is effective to the prevention and improvement of sarcopenia.Citation30,31) We estimated the leucine content in canavalin to be 14.3 g of leucine per 100 g of canavalin, which is approximately 1.5 times higher than that of whey proteins. The high leucine content of canavalin indicates that the intake of canavalin might be more effective than that of whey proteins. In addition, another study reports that the intake of animal proteins such as whey proteins increases carcinogenic risk.Citation32) This risk would be reduced if leucine intake was via canavalin, a plant protein, rather than via whey proteins. These findings indicate the desirability of using canavalin for the development of new processed foods.
In conclusion, we provided a schematic model of canavalin solubility changes induced by concentration changes of divalent cations in the crude extract. Canavalin solubility was affected by the addition of MgCl2 or CaCl2, but not of NaCl. Therefore, divalent cations play an important role in canavalin solubility. We also determined the Cm values for the insolubilization and solubilization phase of canavalin. Thus, our data provide important information regarding the physicochemical properties of canavalin in crude extracts of white sword bean that would be useful for the study of the physiological functions of canavalin and its application in food industry.
Author contributions
KN and YA conceived and designed the experiments. KN performed the experiments. KN and YA analyzed the data and wrote the paper. YA reviewed and edited the manuscript. KN and YA read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Tojuro Iijima Foundation for Food Science and Technology [grant number 11 (individual research, 2014)].
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
References
- Bressani R, Brenes RG, García A, et al. Chemical composition, amino acid content and protein quality of Canavalia spp. seeds. J. Sci. Food Agric. 1987;40:17–23.10.1002/(ISSN)1097-0010
- Siddhurahu P, Becker K. Species/variety differences in biochemical composition and nutritional values of India tribal legumes of genus Canavalia. Nahrung. 2001;45:224–233.10.1002/(ISSN)1521-3803
- Smartt J, editor. Canavalia gladiata (Jacq.) D.C. (sword bean). In: Tropical pulses. London: Longman Group Ltd; 1976. p. 57–59.
- Sasipriya G, Siddhuraju P. Evaluation of growth performance, serum biochemistry and haematological parameters on broiler birds fed with raw and processed samples of Entada scandens, Canavalia gladiata and Canavalia ensiformis seed meal as an alternative protein source. Trop. Anim. Health Prod. 2013;45:811–820.10.1007/s11250-012-0293-z
- Nishizawa K, Masuda T, Takenaka Y, et al. Precipitation of sword bean proteins by heating and addition of magnesium chloride in a crude extract. Biosci. Biotechnol. Biochem. 2016;80:1623–1631.10.1080/09168451.2016.1164587
- Sumner JB, Gralën N, Eriksson-Quensel IB. The molecular weights of urease, canavalin, concanavalin B. Science. 1983;87:395–396.
- Sumner JB. The globulins of the jack bean. Canavalia ensiformis. J. Biol. Chem. 1919;37:137–142.
- Ng JD, Stinchcombe T, Ko T-P, et al. PCR cloning of the full-length cDNA for the seed protein canavalin from the jack bean plant. Canavalia ensiformis. Plant Mol. Biol. 1992;18:147–149.10.1007/BF00018469
- Ng JD, Ko T-P, McPherson A. Cloning, expression, and crystallization of jack bean (Canavalia ensiformis) canavalin. Plant Physiol. 1993;101:713–728.10.1104/pp.101.3.713
- Yamauchi D, Nakamura K, Asahi T, et al. cDNAs for canavalin and concanavalin A from Canavalia gladiata seeds. Nucleotide sequence of cDNA for canavalin and RNA blot analysis canavalin and concanavalin A mRNAs in developing seeds. Eur. J. Biochem. 1988;170:515–520.10.1111/ejb.1988.170.issue-3
- Takei Y, Yamauchi D, Minamikawa T. Nucleotide sequence of the canavalin gene from Canavalia gladiata seeds. Nucleic Acids Res. 1989;17:4384.
- Maruyama N, Adachi M, Takahashi K, et al. Crystal structures of recombinant and native soybean β-conglycinin β homotrimers. Eur. J. Biochem. 2001;268:3595–3604.10.1046/j.1432-1327.2001.02268.x
- Fukuda T, Maruyama N, Salleh MR, et al. Characterization and crystallography of recombinant 7S globulins of adzuki bean and structure—function relationships with 7S globulins of various crops. J. Agric. Food Chem. 2008;56:4145–4153.10.1021/jf072667b
- Sammour RH, Gatehouse JA, Gilroy J, et al. The homology of the major storage protein of jack bean (Canavalia ensiformis) to pea vicilin and its separation from α-mannosidase. Planta. 1984;161:61–70.10.1007/BF00951461
- Gibbs RE, Strongin KB, McPherson A. Evolution of legume seed storage proteins-a domain to common to legumins and vicilins is duplicated in vicilins. Mol. Biol. Evol. 1989;6:614–623.
- Ko TP, Ng JD, Day J, et al. Determination of three crystal structures of canavalin by molecular replacement. Acta Crystallogr. D Biol. Crystallogr. 1993;49:478–489.10.1107/S0907444993004056
- Ko TP, Ng JD, McPherson A. The three-dimensional structure of canavalin from jack bean. Plant Physiol. 1993;101:739–744.
- Ko TP, Day J, McPherson A. The refined structure of canavalin from jack bean in two crystal forms at 2.1 and 2.0 Å resolution. Acta Crystallogr. D Biol. Crystallogr. 2000;56:411–420.10.1107/S0907444900002237
- Ko TP, Kuznetsov YG, Malkin AJ, et al. X-ray diffraction and atomic force microscopy analysis of twinned crystals: rhombohedral canavalin. Acta Crystallogr D Biol Crystallogr. 2001;57:829–839.10.1107/S0907444901003791
- McPherson A. The three-dimensional structure of canavalin at 3.0 Å resolution by X-ray diffraction analysis. J. Biol. Chem. 1980;255:10472–10480.
- Appu Rao AG, Narasinga Rao MS. Binding of Ca(II), Mg(II), and Zn(II) by 7S fraction of soybean proteins. J. Agric. Food Chem. 1976;24:490–494.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.10.1038/227680a0
- Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42.
- Arii Y, Takenaka Y. Initiation of protein association in tofu formation by metal ions. Biosci. Biotechnol. Biochem. 2014;78:86–91.10.1080/09168451.2014.877341
- Tang YT, Gao R, Havranek JJ, et al. Inhibition of bacterial virulence: drug-like molecules targeting the Salmonella enterica PhoP response regulator. Chem. Biol. Drug Des. 2012;79:1007–1017.10.1111/jpp.2012.79.issue-6
- Eknayake S, Jansz ER, Nair BM. Proximate composition, mineral and amino acid content of mature Canavalia gladiate seeds. Food Chem. 1999;66:115–119.10.1016/S0308-8146(99)00041-2
- Arii Y, Takenaka Y. Magnesium chloride concnetratio-dependent formation of tofu-like precipitates with different physicochemical properties. Biosci. Biotechnol. Biochem. 2013;77:928–933.10.1271/bbb.120864
- Maurer RW, Sandler SI, Lehoff AM. Salting-in characteristics of globular proteins. Biophys. Chem. 2011;156:72–78.10.1016/j.bpc.2011.02.002
- Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein syntesis by essential aminoacids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006;291:E381–E387.10.1152/ajpendo.00488.2005
- Yang Y, Breen L, Burd NA, et al. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012;108:1780–1788.10.1017/S0007114511007422
- Kuwata T, Pham AM, MA CY, et al. Elimination of β-lactoglobulin from whey to simulate human milk protein. J. Food Sci. 1985;50:605–609.10.1111/jfds.1985.50.issue-3
- Kurahashi N, Inoue M, Iwasaki M, et al. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective Cohort of Japanese men. Cancer Epidemiol. Biomakers Prev. 2008;17:930–937.10.1158/1055-9965.EPI-07-2681
