Abstract
A cDNA encoding β-mannanase was cloned from Aspergillus niger BCC4525 and expressed in Pichia pastoris KM71. The secreted enzyme hydrolyzed locust bean gum substrate with very high activity (1625 U/mL) and a relatively high kcat/Km (461 mg−1 s−1 mL). The enzyme is thermophilic and thermostable with an optimal temperature of 70 °C and 40% retention of endo-β-1,4-mannanase activity after preincubation at 70 °C. In addition, the enzyme exhibited broad pH stability with an optimal pH of 5.5. The recombinant enzyme hydrolyzes low-cost biomass, including palm kernel meal (PKM) and copra meal, to produce mannooligosaccharides, which is used as prebiotics to promote the growth of beneficial microflora in animals. An in vitro digestibility test simulating the gastrointestinal tract system of broilers suggested that the recombinant β-mannanase could effectively liberate reducing sugars from PKM-containing diet. These characteristics render this enzyme suitable for utilization as a feed additive to improve animal performance.
Graphical abstract
A recombinant β-mannanase from A. niger BCC4525 exhibits high activity. It produces MOS from low-cost biomass and enhance the release of reducing sugars from diet.
Mannan-based polysaccharides are important components of biomass, and function as the building blocks of several plant structural and storage polysaccharides. They are present in the cell walls of softwoods and hardwoods as linear mannans, glucomannans, galactomannans, or galactoglucomannans. In addition, they are present in the bulb and endosperm of some plants. Complete degradation of these complex polymers requires an array of enzymes including endo-β-1,4-mannanase (E.C. 3.2.1.78), β-mannosidase (E.C. 3.2.1.25), α-galactosidase (E.C. 3.2.1.22), and acetylmannanesterase (EC 3.1.1.6).Citation1) Of these, β-mannanase (1,4-β-D-mannanmannanohydrolase; mannan endo 1,4-β-mannosidase) is the key enzyme required for internal cleavage of β-1,4-linkages within the mannan backbone, releasing mannooligosaccharides (MOS) of various lengths as products. Endo-β-1,4-mannanases are usually grouped within the glycosyl hydrolase (GH) family 5 or 26. These families share the same (β/α)8-barrel structure and catalytic mechanism, functioning via a retaining double displacement mechanism.Citation2)
Endo-β-1,4-mannanases have been isolated from various micro-organisms, including gram-positive bacteria, actinomycetes, yeast, and fungi. The optimal pH of these enzymes varies between pH 3 and 7.5, and the optimal temperature ranges from 40 to 75 °C.Citation2) Endo-β-1,4-mannanases with high optimal temperature, thermostability, and broad pH stability are desirable.Citation3,4) For example, several industrial processes are performed at high temperatures, and enzymes capable of working at high temperatures reduce the cooling costs associated with simultaneous saccharification and fermentation and biorefining processes.Citation5)
Over the years, interest in the application of endo-β-1,4-mannanases has increased in industries such as animal feed production,Citation6) detergents,Citation7) and pulp and paper.Citation8) For example, β-mannanase is used to hydrolyze mannan-based polysaccharides into shorter oligosaccharides in animal feed, and this enzyme could be beneficial in some circumstances for broiler gut health.Citation9) Moreover, agricultural wastes are increasingly used as renewable biomass sources for production of value-added feedstocks, chemicals, or biofuels. In various countries like Asia and Africa, agricultural wastes such as palm kernel meal (PKM) and copra meal (CM) contain relatively high levels of mannans. Therefore, efficient hydrolysis of mannans by β-mannanase could help to convert these low-cost biomasses to valuable bioactive compounds.
Recently, an isolate of Aspergillus niger from BIOTEC Culture Collection (BCC) was reported to produce high levels of β-mannanase.Citation9) The crude β-mannanase preparation from this strain had potential value as a feed supplement in an animal field trial. In this study, cDNA encoding β-mannanase was isolated from the fungal strain and cloned into KM71 Pichia pastoris. The resulting recombinant P. pastoris produced β-mannanase with high activity and broad pH stability. In addition, the enzyme exhibited optimal activity and thermostability at high temperatures. The expressed β-mannanase could hydrolyze PKM and CM to produce MOS. Thus, the recombinant enzyme could be valuable in various industrial applications.
Materials and methods
Strains, culture conditions, and plasmids
A. niger BCC4525 was obtained from the BCC, National Center for Genetic Engineering and Biotechnology, Thailand. Escherichia coli DH5α was used as a host for plasmid propagation. Bacterial cultures were grown in Luria–Bertani broth at 37 °C supplemented with ampicillin (100 µg/mL) or zeocin (25 µg/mL), when appropriate. The methylotrophic yeast P. pastoris KM71 (Invitrogen, USA) was used as a host for expression. P. pastoris was cultured in yeast extract peptone dextrose (YEPD) medium [2% (w/v) peptone, 2% (w/v) glucose, and 1% (w/v) yeast extract] or buffered minimal glycerol complex medium (BMGY; Invitrogen, USA) and induced in buffered minimal methanol medium (BMMY; Invitrogen, USA) according to the manufacturer’s manual. The yeast transformants were grown under selective conditions in YEPD medium containing zeocin (100 µg/mL) as a selectable marker. A pPICZαA vector (Invitrogen, USA) containing α factor from S. cerevisiae was used for expression of the recombinant β-mannanase in P. pastoris.
Total RNA isolation and RT-PCR
BCC4525 strain was cultured for five days before cells were harvested by centrifugation at 6000 × g for 15 min. Two grams of mycelia were ground into fine powder in liquid nitrogen using a pestle and mortar. The powdered mycelia were lysed and total RNA was extracted with TRI-Reagent (Molecular Research Center, USA), according to the manufacturer’s instructions. First-strand cDNA was generated from the total RNA using RevertAid H Minus First Strand cDNA Synthesis kit (Fermentas, Lithuania) and poly-dT adaptor (PM1 primer: 5′-CCGGAATTCAAGCTTCTAGAGGATCCTTTTTTTTTTTTTTTT-3′). The cDNA obtained was used as a template in PCR using degenerate primers, which were designed from a conserved internal region of β endo-β-1,4-mannanase gene from Aspergillus species.
Identification and cloning of the putative endo-β-1,4-mannanase from A. niger BCC4525
To obtain a partial sequence of the A. niger BCC4525 endo-β-1,4-mannanase gene, the conserved region of β-mannanase from various Aspergillus species (A. usamii, A. niger, A. aculeatus, and A. oryzae) was used to design degenerate primers ManFW (5′-GGNTAYTTYGCiGGNACiAAYDSiTAYTGG-3′) and ManRV (5′-CCRTAYTCYTCNARiARRCAiGGYTTG-3′). PCR was performed using BCC4525 genomic DNA as a template. PCR conditions were as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles of 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min, with a final incubation at 72 °C for 7 min. The PCR product was subsequently subjected to DNA sequencing (Macrogen Corp., South Korea).
To obtain a full-length β-mannanase cDNA from A. niger BCC4525, the primer pair ManF–EcoRI (5′-GAATGAATTCATGAAGCTTTCCAACGCCCTC-3′) and ManR–Xba (5′-CTAGTCTAGATTAGGCACTATCAATAGCAGCAAC-3′) were designed based on the full-length β-mannanase gene of A. niger and A. usamii because the partial sequence of BCC4525 endo-β-1,4-mannanase gene was found to be most identical to that of A. usamii and A. niger, except for a few base changes. ManF–EcoRI and ManR–Xba primers were used in PCR with A. niger BCC4525 cDNA as a template. PCR conditions were initially denatured at 95 °C for 3 min, followed by 35 cycles of 95 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min with a final incubation at 72 °C for 7 min. The PCR product was subsequently sequenced by Macrogen Corp., South Korea.
The endo-β-1,4-mannanase without the native signal sequence, termed ManF3, from A. niger BCC4525, was amplified by PCR containing ManF3–EcoRI (5′-GAATGAATTCCTGCCGAAAGCCTCCCCTG-3′) and ManR–Xba primers. The resulting PCR product was digested with EcoRI and XbaI and used to replace the EcoRI-XbaI fragment of the pPICZαA plasmid (Invitrogen, USA). The resulting construct, pPICZα–MANF3, contains the β-mannanase coding sequence under the regulation of a methanol-inducible promoter (AOXI) and downstream of an α-factor secretion signal. The plasmid was linearized with PmeI and transformed into KM71 P. pastoris by electroporation according to Invitrogen’s Pichia expression kit manual. The integration of the fusion gene into the yeast genome was confirmed by PCR using genomic DNA as template. A recombinant P. pastoris strain with integrated fusion gene (MANF3) was obtained from a single colony.
Expression of endo-β-1,4-mannanase
To induce production of the recombinant β-mannanase with methanol, the MANF3 strain was cultured in 50 mL BMGY medium at 30 °C by shaking until the culture reached an OD600 of 5–6. Small amount of the culture was diluted 20-fold and used for accurate measurement of OD600 reading. Further, the cell pellets were harvested by centrifugation and resuspended in 10 mL BMMY medium. Absolute methanol was added every 24 h to a final concentration of 3% (v/v) to maintain induction. Culture supernatant was collected by centrifugation.
Protein analysis
BlastX analysis was performed using the web tool hosted at the National Center for Biotechnological Information (http://www.ncbi.nlm.nih.gov/blast/). The InterPro (http://www.ebi.ac.uk/interpro/) and PROSITE databases (http://www.expasy.ch/prosite/) were used to identify a GH family signature sequence within β-mannanase.Citation10) For example, β-mannanase belonging to GH family 5 contains signature sequence [LIV]-[LIVMFYWGA](2)-[DNEQG]-[LIVM-GST]-{SENR}-N-E-[PV]-[RHDNSTLIVFY].Citation10) Alignment of β-mannanase amino acid sequence with other proteins was performed using the ClustalW program (http://align.genome.jp/).Citation11) Theoretical molecular mass and pI of the protein was computed by ExPASy tool (http://web.expasy.org/compute_pi).Citation12) A putative signal sequence was identified using the SignalP prediction program (http://www.cbs.dtu.dk/services/SignalP/).Citation13)
Protein concentrations were determined by measuring absorbance at 595 nm with the BioRad protein assay kit (BioRad, USA) with bovine serum albumin as a standard. Standard SDS–polyacrylamide gel electrophoresis (SDS–PAGE) was performed with 10% polyacrylamide resolving gel.Citation14)
Purification of endo-β-1,4-mannanase
The recombinant β-mannanase was purified by ion-exchange chromatography at 4 °C. Briefly, 0.5 mL of the crude enzyme was loaded into HiTrap DEAE Sepharose FF column (GE Healthcare Life Sciences, USA) pre-equilibrated with 20 mM sodium acetate buffer, pH. 5.5, at a flow rate of 1 mL/min. The column was washed three times with 1 mL of 20 mM sodium acetate buffer at pH. 5.5. After the wash, a linear gradient of 0–1 M NaCl was applied to the column to elute the recombinant enzyme. Fractions containing the purified β-mannanase were detected by SDS–PAGE and mannanase activity was confirmed by standard enzyme assay (below). These fractions were pooled together and subjected to desalting by Amicon® centrifugal filter (Merck Millipore, USA). Finally, the purified β-mannanase was resupended in 20 mM sodium acetate, pH 5.5.
Enzyme assays
Unless stated otherwise, MANF3 activity was assessed by incubation of the enzyme with 5 g/L LBG (Locust Bean Gum) substrate in 0.1 M sodium acetate buffer (pH 5.5) with final volume of 0.4 mL at 70 °C for 10 min. The enzymatic activity was determined using 3,5-dinitrosalicylic acid (the DNS method) as described by Miller.Citation15) One unit of enzyme activity was defined as the amount of enzyme necessary to produce1 μmol of mannose per min. Kinetic experiments were performed under the standard assay conditions stated above, except that 0–10 mg/mL LBG substrate was used. The kinetic parameters were then determined by fitting the initial velocity data to the Michaelis–Menten equation using Kaleida Graph version 3.51 data analysis software (Synergy Software, USA).
Enzyme characterization
The effect of temperature on enzyme activity was assessed in the range 30–100 °C in 100 mM sodium acetate buffer (pH 5.5) for 10 min. Enzyme thermostability was analyzed by measuring the residual activity after preincubating the enzyme at 30–100 °C for 30–120 min. Relative activity was calculated as enzymatic activity at the indicated temperature divided by maximal activity at optimal temperature.
The effect of pH was assessed using different reaction buffers at 0.1 M concentration (sodium acetate buffer pH 3–6, sodium citrate buffer pH 5–6, sodium phosphate buffer pH 6–8, Tris–HCl buffer pH 8–10). The enzyme’s pH stability was tested by preincubating the enzyme in the above-mentioned buffers at 25 °C for 4 h. Following the pre-incubation, a 1:1000 dilution of the enzyme was used to measure the remaining β-mannanase activity under the standard conditions stated above. Relative activity was then calculated as enzymatic activity after 4-h incubation divided by activity at 0-h incubation.
Biomass hydrolysis and thin layer chromatography (TLC)
Non-pretreated PKM or CM (0.05 g) was treated with 6.25 U MANF3 (125 U β-mannanase/g biomass) in 0.1 M sodium acetate buffer at pH 5.5, with a final volume of 1 mL. The hydrolysis was allowed to occur at 50 °C with constant rotation for up to 48 h. Aliquots were collected at various time points and heated at 100 °C for 5 min. Hydrolysed products were separated by TLC on a silica gel plate (Kieselgel 60: Merck, Germany) with n-propanol:ethanol:water (7:1:2 v/v) as a mobile phase. MOS including mannose, mannobiose, mannotriose, mannotetraose, mannopentose, and mannohexose (Megazyme, Ireland) were used as standards. The sugar products were detected by spraying ethanol with 10% sulfuric acid , followed by heating at 100 °C for 5 min.
In vitro digestibility test
The in vitro digestion test was performed as described by Sornlake et al., 2014.Citation9) The in vitro digestion model simulated the three sections of the digestive tract (crop, proventriculus/gizzard, and duodenum, respectively) of chickens fed acorn-based diet. One gram of the corn-based diet was added with 20% non-pretreated PKM in sodium acetate buffer pH 5.7 in triplicate. To these samples, 1.5 mL distilled water (control sample) or enzyme dilution solution at a concentration of 0.5, 1, or 2 U/g diet was added, then the samples were vortexed and incubated at 40 °C for 30 min. After the incubation, 1.5 M HCl was added, followed by 0.5 mL of pepsin solution (Sigma Chemical Co., USA), and the reaction was incubated at 40 °C for 45 min to simulate digestion in the proventriculus and gizzard. For the third step, simulation of the chicken duodenum, 0.45–0.5 mL of 1 M NaHCO3 containing 3.7 mg/mL of pancreatin (Sigma Chemical Co., USA) was added and the reaction was incubated at 40 °C for 2 h. The digested slurry was then centrifuged at 8000 g at 4 °C for 5 min. The reducing sugars released from the digest supernatant were then analyzed using the DNS method.
Gene accession number
The nucleotide sequence of the gene encoding A. niger BCC4525 endo-1,4-β-mannanase was deposited in the Genbank database under the accession number KM096576.
Results
Identification, cloning, and expression of the putative endo-β-1,4-mannanase from A. niger BCC4525
An endo-1,4-β-mannanase cDNA was cloned from A. niger BCC4525, a strain identified as having high β-mannanase activity.Citation9) A partial sequence of this cDNA from degenerate-primed PCR indicated that it was most closely related to the β-mannanases from A. usamii YL-01-78 (accession number HQ839639.1) and A. niger LW-1 (accession number JN811092.1). Further, the full-length cDNA (1152 bp) was obtained by RT-PCR with primers designed based on the β-mannanase genes of A. niger and A. usamii. This sequence was then used for cloning into P. pastoris (below). Subsequently, sequence verification with primers flanking outside the ORF region revealed that the β-mannanase gene from A. niger BCC4525 has two bases that are different from the cDNA fragment obtained from the RT-PCR used for cloning. These differences results in one amino acid change at position 381 (glycine in A. niger BCC4525 and glutamic acid in the P. pastoris clone below). A deduced ORF of β-mannanase from A. niger BCC4525 contains 383 amino acids. Its sequence is identical to that of the β-mannanase gene from A. niger CBS 513.88 (accession number XP001390707) and has two amino acid differences from that of A. usamii (D358 and G381 in A. niger BCC4525 and G358 and D381 in A. usamii). It has a putative molecular mass of 39.22 kDa and a theoretical pI of 4.3. A 21 amino acid signal peptide was predicted at the N-terminus. A. niger BCC4525 putative β-mannanase belongs to the GH family 5 (GH5), with conserved catalytic and disulfide bond forming cysteine residues (Supplemental Information Fig. S1).
BCC4525 β-mannanase cDNA encoding a mature enzyme without its signal sequence, called MANF3, was cloned into a yeast expression vector, and the resulting plasmid was introduced into P. pastoris. Culture media from the selected transformant revealed one major band with an apparent molecular mass of approximately 40 kDa on SDS–PAGE, which is the size expected for recombinant β-mannanase, indicating that the recombinant enzyme was secreted with negligible contaminants. High level induction of recombinant protein was observed after 1–5 days of induction (Supplemental Information Fig. S2); furthermore, a three-day induction was selected for further experiments for convenience and to reduce proteolysis. After three days of induction, the secreted β-mannanase was found to have an activity of 1625 U/mL. The recombinant β-mannanase was purified by anion-exchange chromatography by binding to a DEAE Sepharose column and elution with NaCl gradient. The presence of purified β-mannanase was detected in fractions 12–19 by UV absorbance and SDS–PAGE (Supplemental Information Fig. S3). These fractions were pooled together and subjected to desalting. The mannanase activity present in the eluent was then confirmed by standard enzyme assay. After purification, a specific activity of 802 U/mg was obtained (Table ).
Table 1. Purification of the recombinant β-mannanase from BCC4525.
The kinetic parameters of the recombinant MANF3 enzyme on LBG were determined using 0–10 mg/mL LBG. The Km and Vmax of the enzyme toward LBG were 2.16 ± 0.23 mg/mL and 1533.1 ± 66.2 μM/min/mg protein, respectively. The kcat and kcat/Km values were 996 s−1 and 461 mg−1 s−1 mL, respectively.
Effect of pH and temperature on enzyme activity
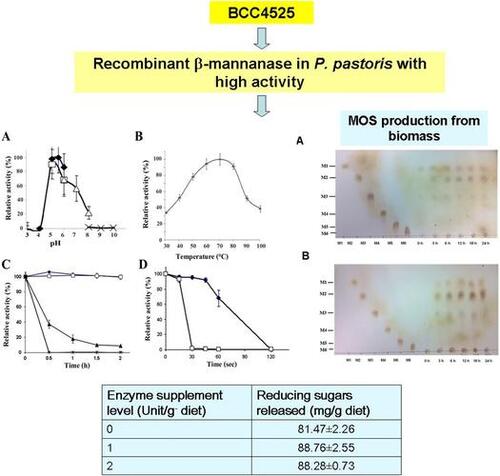
The recombinant enzyme was active at pH 4–7, and optimal activity was observed at pH 5.5 (Fig. (A)). In the absence of any stabilizer, the purified enzyme was highly stable at all pH levels tested (pH 3–10), retaining > 70% of its maximal activity (Supplemental Information Fig. S4).
Fig. 1. Expression of the recombinant MANF3 in KM71 P. pastoris and its characterization. (A) Effect of pH on recombinant BCC4525 β-mannanase activity was determined at different pH values by incubating the enzyme at 70 °C for 10 min in the following buffers; sodium acetate buffer pH 3–6 (closed diamond, ![]()
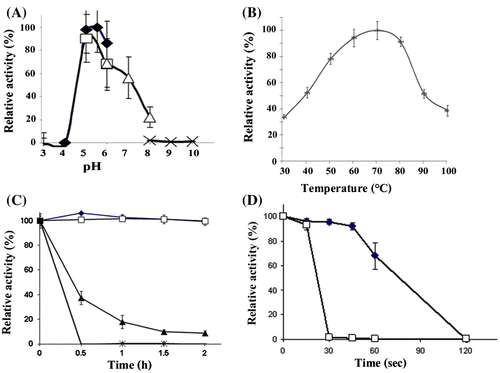
Enzymatic activity was measured between 30 and 100 °C, and the recombinant enzyme exhibited the highest activity at 70 °C. Over 50% of the maximal activity was observed between 40 and 80 °C (Fig. (B)). At 100 °C, the enzyme was functional and exhibited approximately 40% of maximal activity. The enzyme was further found to be highly stable at temperatures up to 60 °C in the absence of stabilizer (Fig. (C)). After incubation at 70 °C, the enzyme retained > 40% activity for 30 min. At 90 °C, the enzyme exhibited > 70% of its maximal activity for up to 60s, whereas at 100 °C, the enzyme was stable for 15s (Fig. (D)).
Hydrolysis of agricultural biomass by the recombinant MANF3
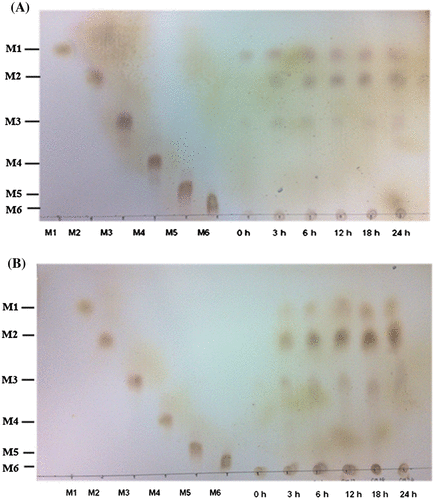
The ability of the recombinant β-mannanase to hydrolyze biomass substrates was examined by TLC. MOS hydrolysis products including mannose (M1), mannobiose (M2), and mannotriose (M3) were detected after untreated PKM and CM were incubated with the enzyme for 3–24 h at 50 °C (Fig. ). The major hydrolysis products obtained from PKM were M1 and M2 with smaller amounts of M3, whereas M2 was the major product of CM hydrolysis. When the reaction was allowed to proceed for up to 48 h, greater amounts of hydrolyzed products were detected as total reducing sugars were obtained (data not shown).
Fig. 2. Hydrolysis of PKM and CM by the recombinant BCC4525 β-mannanase for MOS production. Hydrolysis of PKM (A) and CM (B) was carried out at various time intervals before the reaction products were detected by TLC.
In vitro digestibility
To investigate the potential use of the recombinant mannanase as a supplement for animal feed containing PKM, the in vitro digestibility was assessed. The recombinant β-mannanase was added to the feed and its ability to release reducing sugars under conditions simulating the digestive tract was investigated. Addition of the recombinant enzyme resulted in an increase in reducing sugars. Reducing sugar release was significantly increased from 81.47 mg/g diet to 88.76 or 88.28 mg/g diet in the presence of 1 or 2 U/g β-mannanase (p < 0.05; Table ).
Table 2. The amount of released reducing sugarsTable Footnotea from the digesta fluid obtained from in vitro digestibility test.
Discussion
A cDNA fragment encompassing β-mannanase ORF was isolated from A. niger BCC4525 by RT-PCR with primers designed based on the corresponding sequence of β-mannanase from A. usamii and other A. niger strains. The obtained cDNA without its signal sequence was expressed as a recombinant protein in P. pastoris. Afterward, the cDNA used for cloning was found to contain two nucleotides that were different from the A. niger BCC4525 β-mannanase gene, resulting in one amino acid dissimilarity at position 381 (glycine in A. niger BCC4525 and glutamic acid in the P. pastoris clone). However, it is unlikely that amino acid at this position contributes significantly for enzyme activity and stability, since this position is not conserved and glycine or glutamic acid is found at this position among β-mannanase from Aspergillus species. The recombinant enzyme migrates at approximately 40 kDa in SDS–PAGE, in close agreement with the predicted size (39.2 kDa). The slightly slower migration was caused by N-glycosylation, as shown by a reduction in apparent molecular mass of MANF3 after treatment with PNGase F (Supplemental Information Fig. S2(B)). This glycosylation may contribute to MANF3 stability.Citation16) At present, the N-glycosylation pattern of the recombinant MANF3 cannot be directly compared to that of the native enzyme, since the glycosylation of the native enzyme has not been tested.
High level induction of recombinant protein was observed after 1–5 days of induction. After three days of induction, the secreted β-mannanase was found to have an activity of 1625 U/mL. After purification, the specific activity of the enzyme was 802 U/mg. This value is comparable to that reported by Haung et al. (643 U/mg) for A. niger BK01 β-mannanase expressed in P. pastorisCitation16) and is higher than that reported by Li et al. (212.5 U/mg)Citation17) and Tang et al. (341.3 U/mg)Citation18) for the recombinant A. niger LW-1 β-mannanase and A. usamii YL-01–78 β-mannanase, respectively. The activity of MANF3 is considerably high, which likely proves advantageous for industrial utilization because lower volumes of enzyme will be required and production costs can be decreased.Citation2,19) Quantitative PCR revealed that 1–2 copies of the recombinant construct integrated into the host’s genome (data not shown); therefore, high mannanase activity is not because of high gene copy number. The recombinant enzyme exhibited kcat and kcat/Km values of 996 s−1 and 461 mg−1 s−1 mL, respectively. This kcat value is higher than that reported earlier by Do et al. (330 s−1) for A. niger BK01 mannanaseCitation20), but was lower than that reported by Huang et al. (1803 s−1) with mannanase from the same organism (A. niger BK01).Citation16) This discrepancy may stem from differences in induction or assay conditions. The kcat/Km value revealed that the MANF3 catalytic efficiency was relatively high compared to mannanases from micro-organisms. For comparison, Do et al.Citation20) and Huang et al.Citation16) reported the kcat/Km value of A. niger BK01 mannanase to be 165 mg−1s−1 mL and 2279 mg−1s−1 mL, respectively. Another mannanase from A. niger achieved a kcat/Km value of 120.75Citation19), whereas mannanases from A. nidulan,Citation21) Trichoderma reseii,Citation21) and Reinekea sp.Citation22) exhibited kcat/Km values of 70, 400, and 329 mg−1 s−1 mL, respectively. The high catalytic efficiency of the recombinant MANF3 most likely contributed to its high unit activity.
In this study, the MANF3 mannanase was found to exhibit high activity at pH 4–7. This activity at acidic-to-neutral pH, coupled with its broad pH stability, suggests that the enzyme may function well in the gastrointestinal tract of broiler chicks and swine if used as a feed supplement. In addition, the optimal temperature of the recombinant mannanase expressed in P. pastoris was 70 °C, and the enzyme was thermostable up to 70 °C. These properties are comparable to those reported by Li et al.Citation17) and Do et al.Citation20) for recombinant β-mannanase. The high thermostability of MANF3 shows that the recombinant mannanase is most likely suitable for animal feed-pelleting processes during which high temperature is usually applied.
The recombinant MANF3 may be very useful in the food and feed industry. PKM and CM can be used as low-cost feed ingredients. PKM and CM hydrolysis by the recombinant enzyme was carried out at 50 °C, which allowed the enzyme to already exhibit approximately 80% of its maximal activity while posted a lower risk of hazard than hydrolysis at 70 °C (the enzyme’s optimal working temperature). Our results showed that the recombinant β-mannanase could efficiently hydrolyze PKM and CM to release various short-chain MOS, which can function as an energy source for animals. Most importantly, M2 and M3 produced by the enzyme can most likely be used as a prebiotic to improve animal gut health by promoting growth of beneficial microorganisms similarly to previous reports by Baurhoo et al. and Kovacs-Nolan et al.Citation23,24). The different types of MOS obtained after hydrolyzing PKM or CM (PKM hydrolysis yields mostly M1 and M2, whereas CM hydrolysis yields mostly M2) is most likely because of the different mannan composition of PKM and CM. PKM tends to contain a higher percentage of linear mannan, but a lower percentage of galactomanan than CM.Citation25,26) Further study, including pretreatment of PKM and CM substrates and field trials with broiler chicks, should be performed to assess the efficacy of MOS obtained from the low-cost biomass by recombinant β-mannanase to increase feed conversion efficiency and energy utilization in vivo. The in vitro digestibility test indicated that supplementing feed with the enzyme could improve energy metabolism. Supplementation of broiler diets containing 20% PKM with β-mannanase increased release of reducing sugars. Because PKM is considered inexpensive, our results suggested that recombinant β-mannanase may represent a beneficial supplement for animal feed containing 20% PKM and may reduce the cost of animal feed. Exploring the optimal ratio of PKM and enzyme supplement could improve the growth rate of broilers.Citation25)
In conclusion, a recombinant β-mannanase from the BCC4525 strain exhibits high activity toward its substrates, and characteristics suited to various industrial uses, particularly the food and feed industry. The recombinant enzyme was able to hydrolyze PKM and CM biomass to yield multiple beneficial MOS, which can be metabolized by animal and/or further utilized as prebiotics. The ease and high yield of this enzyme preparation render this recombinant β-mannanase ideal for large-scale production to help improve animal performance. Furthermore, the recombinant BCC4525 enzyme is well suited for other industrial applications such as hydrolysis of coffee extract, as spent coffee grounds has an approximate pH of 5.
Author contributions
PH designed research, performed experiments, and wrote the manuscript, WP, WT, WS, and KS performed experiments, PM and ST designed research and advised on research. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the National Center for Genetic Engineering and Biotechnology under [P-09-00292]; the National Science and Technology Development Agency [P-13-50429] and the Young Scientist and Technologist Program (YSTP).
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1230003.
TBBB_1230003_Supplementary_Material.pdf
Download PDF (363 KB)Acknowledgments
We thank Ms. Peerada Promdonkoy for technical assistance and Dr. Phillip Shaw for critically editing the manuscript. Financial support from National Center for Genetic Engineering and Biotechnology, Thailand, National Science and Technology Development Agency, and Young Scientist and Technologist Program (YSTP) is greatly appreciated.
References
- Moreira LR, Filho EX. An overview of mannan structure and mannan-degrading enzyme systems. Appl. Microbiol. Biotechnol. 2008;79:165–178.10.1007/s00253-008-1423-4
- Chauhan PS, Puri N, Sharma P, et al. Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biotechnol. 2012;93:1817–1830.10.1007/s00253-012-3887-5
- Duffaud GD, McCutchen CM, Leduc P, et al. Purification and characterization of extremely thermostable β-mannanase, β-mannosidase, and α-galactosidase from the hyperthermophilic eubacterium Thermotoga neapolitana 5068. Appl. Environ. Microbiol. 1997;63:169–177.
- Promdonkoy P, Tang K, Sornlake W, et al. Expression and characterization of Aspergillus thermostable phytases in Pichia pastoris. FEMS Microbiol. Lett. 2009;290:18–24.10.1111/fml.2008.290.issue-1
- Turner P, Mamo G, Karlsson EN. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 2007;6:9.10.1186/1475-2859-6-9
- Sae-Lee N. The production of fungal mannanase, cellulose and xylanase using palm kernel meal as a substrate. Walailak J. Sci. Tech. 2007;4:67–82.
- Takeda N, Hirasawa K, Uchimura K, et al. Purification and enzymatic properties of a highly alkaline mannanase from alkaliphilic Bacillus sp. strain JAMB-750. J. Biol. Macromol. 2004;4:67–74.
- Gubitz GM, Haltrich D, Latal B, et al. Mode of depolymerisation of hemicellulose by various mannanases and xylanases in relation to their ability to bleach softwood pulp. Appl. Microbiol. Biotechnol. 1997;47:658–662.
- Sornlake W, Matetaviparee P, Rattanaphan N, et al. β-Mannanase production by Aspergillus niger BCC4525 and its efficacy on broiler performance. J. Sci. Food Agric. 2013;93:3345–3351.10.1002/jsfa.2013.93.issue-13
- Falquet L, Pagni M, Bucher P, et al. The PROSITE database, its status in 2002. Nucleic Acids Res. 2002;30:235–238.10.1093/nar/30.1.235
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948.10.1093/bioinformatics/btm404
- Gasteiger E, Hoogland C, Gattiker A, et al. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The proteomics protocols handbook. Humana Press; 2005. p. 571–607.10.1385/1592598900
- Bendtsen JD, Nielsen H, von Heijne G, et al. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–795.10.1016/j.jmb.2004.05.028
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.10.1038/227680a0
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428.10.1021/ac60147a030
- Huang J, Chen C, Huang C, et al. Improving the specific activity of β-mannanase from Aspergillus niger BK01 by structure-based rational design. Biochim. Biophys. Acta. 2014;1844:663–669.10.1016/j.bbapap.2014.01.011
- Li J-F, Zhao S-G, Tang C-D, et al. Cloning and functional expression of an acidophilic β-mannanase gene (Anman5A) from Aspergillus niger LW-1 in Pichia pastoris. J. Agric. Food Chem. 2012;60:765–773.10.1021/jf2041565
- Tang C-D, Guo J, Li J-F, et al. Enhancing expression level of an acidophilic β-mannanase in Pichia pastoris by double vector system. Ann. Microbiol. 2014;64:561–569.10.1007/s13213-013-0689-7
- Zhao W, Zheng J, Zhou H. A thermotolerant and cold-active mannan endo-1,4-β-mannosidase from Aspergillus niger CBS 513.88: constitutive overexpression and high-density fermentation in Pichia pastoris. Biores. Tech. 2011;102:7538–7547.10.1016/j.biortech.2011.04.070
- Do BC, Dang TT, Berrin JG, et al. Cloning, expression in Pichia pastoris, and characterization of a thermostable GH5 mannan endo-1,4-β-mannosidase from Aspergillus niger BK01. Microb. Cell Fact. 2009;8:59.
- Rosengren A, Reddy S, Sjöberg JS, et al. An Aspergillus nidulans β-mannanase with high transglycosylation capacity revealed through comparative studies within glycosidase family 5. Appl. Microbiol. Biotechnol. 2014;98:10091–10104.10.1007/s00253-014-5871-8
- Hakamada Y, Ohkubo Y, Ohashi S. Purification and characterization of β-Mannanase from Reinekea sp. KIT-YO10 with transglycosylation activity. Biosci. Biotechnol. Biochem. 2014;78:722–728.
- Baurhoo B, Phillip L, Ruiz-Feria CA. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007;86:1070–1078.10.1093/ps/86.6.1070
- Kovacs-Nolan J, Kanatani H, Nakamura A, Ibuki M, Mine Y. β-1, 4-mannobiose stimulates innate immune responses and induces TLR4-dependent activation of mouse macrophages but reduces severity of inflammation during endotoxemia in mice. J. Nutr. 2013;143:384–391.10.3945/jn.112.167866
- Balasubramaniam K. Polysaccharides of the kernel of maturing and matured coconuts. J. Food Sci. 1976;41:1370–1373.10.1111/jfds.1976.41.issue-6
- Sundu B, Kumar A, Dingle J. Palm kernel meal in broiler diets: effect on chicken performance and health. Worlds Poult. Sci. J. 2006;62:316–325.10.1079/WPS2005100
