Abstract
Bioactivity-guided isolation of Opuntia ficus-indica (Cactaceae) seeds against ethanol-treated primary rat hepatocytes yielded six lignan compounds. Among the isolates, furofuran lignans 4−6, significantly protected rat hepatocytes against ethanol-induced oxidative stress by reducing intracellular reactive oxygen species levels, preserving antioxidative defense enzyme activities, and maintaining the glutathione content. Moreover, 4 dose-dependently induced the heme oxygenase-1 expression in HepG2 cells.
Opuntia ficus-indica (L.) Mill. (Cactaceae) is a perennial plant known as prickly pear or nopal. This cactus is considered to be native to Mexico, but is currently cultivated in Mediterranean countries, Latin America, Southern Asia, and several oceanic islands with warm climates.Citation1) Previous studies on the chemical composition of O. ficus-indica revealed the presence of alkaloids, flavonoids, terpenoids, polysaccharides, and organic acids.Citation2–4) Recent studies have reported that the extract of O. ficus-indica showed antioxidant, antiulcer, neuroprotective, and hepatoprotective activities.Citation5–8) To the best of our knowledge, however, the hepatoprotective chemical constituents in this plant against ethanol-induced hepatotoxicity have not been previously reported. Thus, in this study, we attempted to investigate the hepatoprotective constituents of O. ficus-indica using primary rat hepatocytes and HepG2 cells as screening tools.
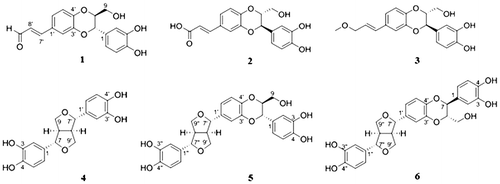
Reactive oxygen species (ROS) generated from the ethanol metabolism process induces oxidative stress in hepatocytes and eventually cause liver damage.Citation9) Any substance possessing antioxidant properties against ethanol-induced liver toxicity is considered to be a potential therapeutic agent for preventing the progression of alcoholic liver disease (ALD). In the course of searching for antioxidant agents from natural products using ethanol-treated rat primary hepatocytes, 80% ethanol extract of O. ficus-indica seeds showed hepatoprotective activity. Bioassay-guided isolation of O. ficus-indica seeds resulted in the isolation of six lignan compounds that included 7S,8S-isoamericanin A (1), 7R,8R-isoamericanoic acid A (2), 7R,8R-9′-O-methylisoamericanol A (3), 3,3′-bisdemethylpinoresinol (4), 7S,8S-isoprincepin (5), and 7S,8S-princepin (6) (Fig. ).Citation10–15) Among the isolates, 2 was reported for the first time from this plant. The presence of 1 and 3−6 in this plant had been previously reported in a Korean domestic patent; however, their absolute configurations of the structures were first identified in this study by comparison of their NMR and CD spectroscopic data with literature values (Table S1, S2, and Fig. S1).Citation10–15)
All isolates (1−6) were evaluated for their hepatoprotective activities against ethanol-treated primary cultured rat hepatocytes (Table S3). Quercetin was used as a positive control because it is a well-known hepatoprotective phytochemical.Citation16) The isolated compounds 1−6 showed higher protection than that of the positive control. Especially, 4–6, furofuran-type lignans consisting of the phenyl propanoid dimer linked by a C8-C8’ covalent bond, exhibited significant protective activities against ethanol-induced hepatotoxicity. The furofuran lignans 4–6 showed almost the same protective activity (EC50; 13.7 μM, 18.6 μM, and 22.2 μM, respectively). We used the most abundant compound 4 that is a little stronger than those of compounds 5 and 6 for the further investigation.
Because ethanol-induced hepatotoxicity is caused by oxidative stress generated during ethanol metabolism, we examined the anti-oxidative activities of compounds 4–6. First, the effects of 4–6 on the content of intracellular peroxides were measured with a fluorimeter using 2,7-DCF-DA fluorescent dye (Fig. (A)).Citation9) The ROS levels increased by ethanol insult were decreased by the pretreatment of 4–6. Additionally, to confirm the ROS scavenging activity of 4, the intracellular peroxidation of HepG2 cells was assessed by flow cytometry (Fig. (B)).Citation17) Our results showed that the increased intracellular ROS production by ethanol treatment (18.5%) compared to the control group (4.7%) was effectively reduced by the pretreatment of 4 (10.0%). Moreover, the effects of 4–6 on antioxidant enzyme activities and GSH content in ethanol-treated primary rat hepatocytes were evaluated (Table ).Citation9) GR and GSH-PX activities, reduced by ethanol insult, were recovered by the treatment with 4–6. The reduced content of GSH and increased ratio of GSSG/total GSH induced by ethanol were also reversed by the treatment with 4–6. These results suggest that 4–6 effectively prevented ethanol-induced GSH depletion by restoring the activities of the antioxidant enzymes GSH-PX and GR. Especially, 4, which exhibited the most remarkable change in reducing the cellular peroxide levels and restoring the antioxidant defense enzymes and total GSH content, has been suggested as the most powerful contributor to the anti-oxidative properties of O. ficus-indica seeds.
Fig. 2. Effects of compounds 4–6 on intracellular ROS production and the relationship between compound 4 and HO-1 expression.
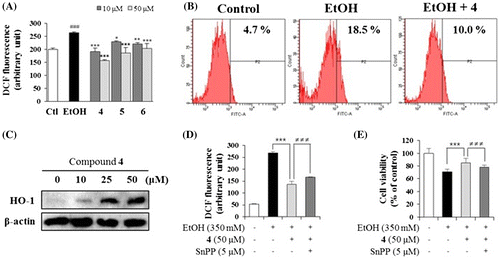
Table 1. The effects of 4–6 on the activities of antioxidant enzymes and the glutathione level in ethanol-treated primary rat hepatocytes.
To determine whether compound 4, the substance showing the most potent hepatoprotective and antioxidant properties, regulates HO-1 to protect cells against oxidative damages, we performed western blot analysis (Fig. (C)). HO-1, a rate-limiting enzyme in heme catabolism, is known as a stress-response protein which has a fundamental role against oxidative stress by degrading heme into metabolites.Citation18) We observed that 4 significantly induced HO-1 expression in HepG2 cells in a concentration-dependent manner. In addition, our data showed that the inhibition of the HO-1 activity by HO-1 inhibitor, SnPP, reversed the antioxidative (Fig. (D)) and hepatoprotective (Fig. (E)) effect of 4 against ethanol-treated HepG2 cells. These results indicate that the antioxidative and hepatoprotective effect of 4, at least partly, is mediated through the expression of HO-1. This is the first report showing that a furofuran lignan exhibits antioxidative and hepatoprotective effects by inducing HO-1 protein expression in HepG2 human liver cells. In-depth study is needed to fully elucidate the hepatoprotecive mechanism by furofuran lignan and further in vivo evaluations are demanded.
Author contributions
Jung Wha Kim carried out the experiments with assistance from Hyeon Woo Kim. Jung Wha Kim prepared the manuscript. Heejung Yang and Hong Pyo Kim contributed analysis and discussion. Jung Wha Kim and Sang Hyun Sung designed the project and reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which was funded by the Ministry of Science, ICT and Future Planning [NRF-2015M3A9A5030733].
Supplemental data
The supplemental data for this paper is available online at http://dx.doi.org/10.1080/09168451.2016.1234930.
TBBB_1234930_Supplementary_Material.docx
Download MS Word (228.2 KB)References
- Ramadan MF, Mörsel JT. Recovered lipids from prickly pear [Opuntia ficus-indica (L.) Mill] peel: a good source of polyunsaturated fatty acids, natural antioxidant vitamins and sterols. Food Chem. 2003;83:447–456.10.1016/S0308-8146(03)00128-6
- Saleem M, Kim HJ, Han CK, et al. Secondary metabolites from Opuntia ficus-indica var. saboten. Phytochemistry. 2006;67:1390–1394.10.1016/j.phytochem.2006.04.009
- Lee EH, Kim HJ, Song YS, et al. Constituents of the stems and fruits of Opuntia ficus-indica var. saboten. Arch Pharm Res. 2003;26:1018–1023.10.1007/BF02994752
- Alarcon-Aguilar FJ, Valdes-Arzate A, Xolalpa-Molina S, et al. Hypoglycemic activity of two polysaccharides isolated from Opuntia ficus-indica and O. streptacantha. Proc West Pharmacol Soc. 2003;46:139–142.
- Lee MH, Kim JY, Yoon JH, et al. Inhibition of nitric oxide synthase expression in activated microglia and peroxynitrite scavenging activity by Opuntia ficus indica var. saboten. Phytother Res. 2006;20:742–747.10.1002/(ISSN)1099-1573
- Galati EM, Mondello MR, Giuffrida D, et al. Chemical characterization and biological effects of Sicilian Opuntia ficus indica (L.) mill. Fruit juice: antioxidant and antiulcerogenic activity. J Agric Food Chem. 2003;51:4903–4908.10.1021/jf030123d
- Galati EM, Mondello MR, Lauriano ER, et al. Opuntia ficus indica (L.) Mill. fruit juice protects liver from carbon tetrachloride-induced injury. Phytother Res. 2005;19:796–800.10.1002/(ISSN)1099-1573
- Alimi H, Hfaeidh N, Mbarki S, et al. Evaluation of Opuntia ficus indica f. inermis fruit juice hepatoprotective effect upon ethanol toxicity in rats. Gen Physiol Biophys. 2012;31:335–342.10.4149/gpb_2012_038
- Kim JW, Yang H, Cho N, et al. Hepatoprotective constituents of Firmiana simplex stem bark against ethanol insult to primary rat hepatocytes. Pharmacogn Mag. 2015;11:55–60.
- Kamiya K, Tanaka Y, Endang H, et al. Chemical constituents of Morinda citrifolia fruits inhibit copper-induced low-density lipoprotein oxidation. J Agric Food Chem. 2004;52:5843–5848.10.1021/jf040114k
- Jin CB, Kim HJ, Lee, YS, et al., Korea Institute of Science and Technology. Liver toxicity disorder composition comprising an extract from the seed of Opuntia ficus-indica var. saboten and compounds isolated therefrom. Repub. Korean Kongkae Taeho Kongbo patent KR2009086817. 2009 Aug 14.
- Lin CF, Ni CL, Huang YL, et al. Lignans and anthraquinones from the fruits of Morinda citrifolia. Nat Prod Res. 2007;21:1199–1204.10.1080/14786410601132451
- Kim NC, Graf TN, Sparacino CM, et al. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org Biomol Chem. 2003;1:1684–1689.10.1039/b300099k
- Waibel R, Benirschke G, Benirschke M, et al. Sesquineolignans and other constituents from the seeds of Joannesia princeps. Phytochemistry. 2003;62:805–811.10.1016/S0031-9422(02)00357-6
- Casabuono AC, Pomilio AB. Lignans and a stilbene from Festuca argentina. Phytochemistry. 1994;35:479–483.10.1016/S0031-9422(00)94786-1
- Liu S, Hou W, Yao P, et al. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicol In Vitro. 2010;24:516–522.10.1016/j.tiv.2009.03.006
- Tavakkol-Afshari J, Brook A, Mousavi SH. Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem Toxicol. 2008;46:3443–3447.10.1016/j.fct.2008.08.018
- Tang Y, Tian H, Shi Y, et al. Quercetin suppressed CYP2E1-dependent ethanol hepatotoxicity via depleting heme pool and releasing CO. Phytomedicine. 2013;20:699–704.10.1016/j.phymed.2013.03.010