Abstract
We examined the inhibitory effects of HAQ (His-Ala-Gln) peptide on type-1 allergy in vitro and in vivo. HAQ peptide inhibited β-hexosaminidase release and intracellular Ca2+ levels of rat basophilic leukemia RBL-2H3 cells. Oral administration of a HAQ peptide-added diet (1 mg/mouse/administration) to C3H/HeJ mice for 14 days led to significant suppression of allergic symptoms, but did not reduce allergen-specific IgE or IgG1.
Type-1 allergy, as typified by food and pollen allergies, is defined as a hypersensitivity reaction, and its frequency is increasing worldwide.Citation1) There is a need to develop therapeutic agents that either prevent sensitization to allergens or suppress the allergic response after initiation. It has been reported that polyphenolic compounds such as catechin show anti-allergic effects,Citation2–6) but there have been few reports about peptides. Our group has previously reported that HAQ (His-Ala-Gln) tripeptide, which is present in CE90GMM, a peptide mixture derived from milk casein inhibits degranulation of RBL-2H3 cells.Citation7)
In the present study, we examined the anti-allergic effects of HAQ peptide in vitro and in vivo. To evaluate the effects in vivo, we performed oral administration of HAQ peptide to a mouse model of type-1-mediated food allergy to lysozyme from hen egg white (LHE).
Artificially synthesized HAQ peptide (>95% purity) was purchased from Sigma-Aldrich (St. Louis, MO, USA). RBL-2H3 cells were maintained in Dulbecco’s modified Eagle’s medium (Nacalai Tesque, Tokyo, Japan) with 10% (v/v) fetal calf serum (Sigma-Aldrich), 100 U/mL penicillin (Nacalai Tesque), and 100 μg/mL streptomycin (Nacalai Tesque) at 37 °C in a humidified atmosphere containing 5% CO2.
Ca2+ is one of the major second messengers in intracellular signaling. It is known that intracellular Ca2+ ([Ca2+]i) in mast cells and basophils increases through signaling after cross-linkage of antigens to FcεRI through IgE for degranulation. Thus, the degranulation-suppressing effect of HAQ peptide on [Ca2+]i was examined using fluo-3 AM. [Ca2+]i was measured using Calcium Kit-Fluo 3 (Dojindo Laboratories, Kumamoto, Japan) according to the method of Ishida et al.Citation8) Furthermore, cytotoxicity of HAQ peptide in RBL-2H3 cells was examined using cell count reagent SF (Nacalai Tesque) according to the manufacturer’s instructions. Fig. (A) shows the inhibitory effects of HAQ peptide on antigen-induced β-hexosaminidase release from RBL-2H3 cells. HAQ peptide significantly and dose-dependently reduced β-hexosaminidase release. A cell viability assay showed that HAQ peptide was not cytotoxic within the tested concentrations (Fig. (B)). [Ca2+]i in RBL-2H3 cells rapidly increased and was sustained for 15 min after stimulation with antigen, whereas the elevation of [Ca2+]i was suppressed in the presence of HAQ peptide. These results indicate that HAQ peptide reduces degranulation by suppressing the elevation of [Ca2+]i due to stimulation by the antigen–antibody interaction (Fig. (C)). Our group has previously reported that HAQ tripeptide from milk casein inhibits degranulation of RBL-2H3 cells.Citation7) The present results of [Ca2+]i in RBL-2H3 cells are consistent with our previous report.Citation7)
Fig. 1. Effects of HAQ peptide on the release of β-hexosaminidase (A), cell viability (B), and [Ca2+]i (C) of RBL-2H3 cells. (A) DNP-specific IgE-sensitized RBL-2H3 cells were challenged with DNP-HSA for 30 min. HAQ peptide was added at 10 min before antigen challenge. (B) Cell viability was measured using cell count reagent SF solution after various concentrations of HAQ peptide were added to anti-DNP IgE-sensitized RBL-2H3 cells followed by stimulation with DNP-HSA. (C) [Ca2+]i was measured by Calcium Kit-Fluo-3. Anti-DNP IgE-sensitized cells were incubated with Fluo-3 AM for 1 h and then incubated with 500 μM HAQ peptide or PBS for 10 min. Then, the treated cells were stimulated with DNP-HSA, and the fluorescence intensity was measured. (○), non-HAQ peptide-treated cells not sensitized with anti-DNP IgE; (▲), HAQ peptide-treated cells stimulated with antigen; (●), non-HAQ peptide treated cells stimulated with antigen.
![Fig. 1. Effects of HAQ peptide on the release of β-hexosaminidase (A), cell viability (B), and [Ca2+]i (C) of RBL-2H3 cells. (A) DNP-specific IgE-sensitized RBL-2H3 cells were challenged with DNP-HSA for 30 min. HAQ peptide was added at 10 min before antigen challenge. (B) Cell viability was measured using cell count reagent SF solution after various concentrations of HAQ peptide were added to anti-DNP IgE-sensitized RBL-2H3 cells followed by stimulation with DNP-HSA. (C) [Ca2+]i was measured by Calcium Kit-Fluo-3. Anti-DNP IgE-sensitized cells were incubated with Fluo-3 AM for 1 h and then incubated with 500 μM HAQ peptide or PBS for 10 min. Then, the treated cells were stimulated with DNP-HSA, and the fluorescence intensity was measured. (○), non-HAQ peptide-treated cells not sensitized with anti-DNP IgE; (▲), HAQ peptide-treated cells stimulated with antigen; (●), non-HAQ peptide treated cells stimulated with antigen.](/cms/asset/a74b9ae9-6c7a-4d04-8ea3-b79ae8716f30/tbbb_a_1243984_f0001_b.gif)
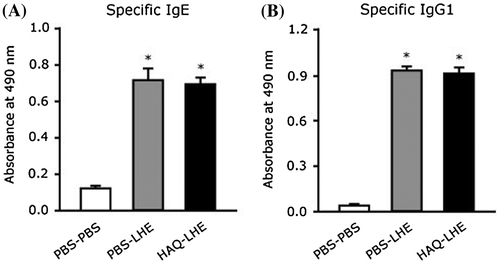
To investigate the effects of HAQ peptide on the antigen-specific immune response and allergic reaction in vivo, we performed animal experiments using a murine food allergy model. The experimental design was in accordance with the guidelines for animal experimentation and was approved by the Animal Experimental Committee of the University of Kochi (authorization number: 2014-005). In this study, we selected C3H/HeJ mouse which is used as a murine food allergy model.Citation9,10) C3H/HeJ mice (5 weeks old, female, weighing 15–20 g) were randomly assigned to HAQ–LHE (n = 8), PBS–PBS (n = 5), and PBS–LHE (n = 9) groups. PBS–PBS and PBS–LHE groups were orally administered phosphate-buffered saline (PBS; pH 7.0) alone. HAQ–LHE and PBS–LHE groups were initially injected intraperitoneally with 100 μg LHE (Wako Pure Chemicals, Osaka, Japan) and 4 mg aluminum hydroxide in 0.2 mL PBS on day 1. LHE was then reduced to 50 μg and injected intraperitoneally with 4 mg aluminum hydroxide in 0.2 mL PBS on day 8. To assess the sensitization, blood was obtained from the orbital veins of mice under light anesthesia 11 days after the intial injection and subjected to enzyme-linked immunosorbent assays (ELISAs). The HAQ–LHE group was orally administered HAQ peptide (1 mg/mouse/administration/day) in 0.2 mL PBS by gavage throughout the experimental period for 14 days. The PBS–PBS group received the vehicle alone. Mean body weights did not differ between the three groups during a survey period. To determine whether the development of an antigen-specific immune response could be modulated by oral administration of HAQ peptide, specific IgE and IgG1 levels in mouse serum were evaluated by ELISAs as described previously.Citation11) The amount of LHE-specific IgE produced by mice injected with LHE was significantly greater than that produced by the PBS–PBS group, whereas a significant difference in LHE-specific IgE levels was not observed between HAQ–LHE and PBS–LHE groups (Fig. (A)). The reactivity of IgG1 to LHE was also measured in sera from sensitized mice (Fig. (B)). The amount of IgG1-specific LHE produced by mice injected with LHE was significantly greater than that produced by the PBS–PBS group, whereas a significant difference in LHE-specific IgG1 levels was not observed between HAQ–LHE and PBS–LHE groups.
Fig. 2. LHE-specific IgE (A) and IgG1 (B) levels in sera of control and HAQ peptide-fed C3H/HeJ mice. □, unsensitized, PBS orally administered group (n = 5); ■, LHE-sensitized, PBS orally administered group (n = 9);
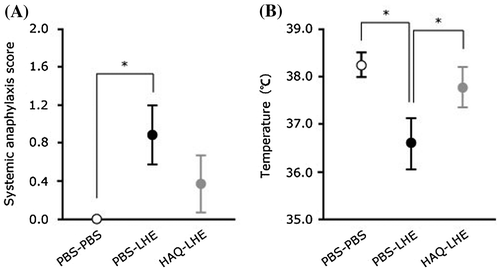
We next evaluated the effect of orally administered LHE on suppression of allergic reactions in a murine model. The score assessment commonly increases and whole body temperature is commonly reduced during systemic anaphylaxis.Citation9,11) Score assessment and body temperature measurement were therefore conducted to assess anti-allergic effects in mice. HAQ–LHE and PBS–LHE groups were orally administered 10 mg LHE in 0.5 mL PBS, and then score assessment and rectal temperature measurement were performed to evaluate allergic symptoms. The PBS–PBS group was orally administered 0.5 mL PBS alone. Score assessments were performed at 20 min after challenge with LHE using the scoring system as described by Li et al.Citation9) To evaluate body temperature, rectal temperature was measured at 30 min after oral administration of LHE with a digital temperature indicator (SN3502; AS ONE Corporation, Osaka, Japan). In the score assessment, the PBS–LHE group (0.9 ± 0.3) was significantly higher than the PBS–PBS group (0.0 ± 0.0), but not the HAQ–LHE group (0.4 ± 0.3) (Fig. (A)). In terms of rectal temperatures, the PBS–LHE group (36.6 ± 0.5) had a significant decrease in body temperature compared with the PBS–PBS (38.2 ± 0.3) and HAQ–LHE (37.8 ± 0.4) groups (Fig. (B)). Our present data demonstrate that administration of HAQ peptide to sensitized mice suppresses the consistent allergic symptoms induced by antigen stimulation. These results indicate that HAQ peptide also has anti-allergic effects in vivo as well as in vitro.Citation7) In contrast, the antigen-specific IgE and IgG1 induced by administration of HAQ peptide to sensitized mice were not lower compared with administration of PBS to sensitized mice. These results may indicate another immunosuppressive pathway shifting from a Th2-dominated to Th1-dominated immune response of specific antibody-producing cells, because HAQ peptide did not suppress IgE and IgG1 production.
Fig. 3. Allergic symptoms after oral challenge in mice presensitized in the presence of HAQ peptide. ○, unsensitized, PBS orally administered group (n = 5); ●, LHE-sensitized, PBS orally administered group (n = 9);
In conclusion, we demonstrated that the inhibitory effect of HAQ peptide results from suppressed mobilization of [Ca2+]i. In addition, continuous administration of HAQ peptide could not reduce allergen-specific IgE and IgG1 production, but suppressed the mild allergic symptoms in a murine model of food allergy.
Author contributions
Mamoru Tanaka, Hiroyuki Watanabe, Yoshinobu Yoshimoto, Hana Kozai, and Takeaki Okamoto conceived and designed the experiments. Mamoru Tanaka, and Takeaki Okamoto performed the experiments. Mamoru Tanaka, Hiroyuki Watanabe, Yoshinobu Yoshimoto, Hana Kozai, and Takeaki Okamoto analyzed data. Mamoru Tanaka, Hiroyuki Watanabe, and Takeaki Okamoto contributed reagents/materials/analysis tools. Mamoru Tanaka, Yoshinobu Yoshimoto, and Takeaki Okamoto wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by JSPS KAKENHI [grant number 25750051].
Acknowledgments
The authors thank Junko Hirano and Rina Hirata for their technical assistance.
References
- Kagan RS. Food allergy: An overview. Environ Health Perspect. 2003;111:223–225.10.1289/ehp.5702
- Kanda T, Akiyama H, Yanagida A, et al. Inhibitory effects of apples polyphenol on induced histamine release from RBL-2H3 cells and rat mast cells. Biosci Biotechnol Biochem. 1998;62:1284–1289.10.1271/bbb.62.1284
- Alexandrakis M, Singh L, Boucher W, et al. Differential effect of flavonoids on inhibition of secretion and accumulation of secretory granules in rat basophilic leukemia cells. Int. J Immunopharmacol. 1999;21:379–390.10.1016/S0192-0561(99)00018-1
- Fujimura Y, Tachibana H, Maeda-Yamamoto M, et al. Antiallergic tes catechin, (−)-epigallocatechin-3-O-(3-O-methyl)-gallate, suppresses FcepsilonRI expression in human basophilic KU812 cells. J Agric Food Chem. 2002;50:5729–5734.10.1021/jf025680z
- Inoue T, Sugimoto Y, Masuda H, et al. Antiallergic effect of flavonoid glycosides obtained from Mentha piperita L. Biol Pharm Bull. 2002;25:256–259.10.1248/bpb.25.256
- Katayama S, Mine YJ. Quillaga saponin can modulate ovalbumin-induced IgE allergic responses through regulation of Th1/Th2 balance in a murine model. Agric Food Chem. 2006;54:3271–3276.10.1021/jf060169h
- Tanaka M, Yamagishi K, Sugahara T, et al. Impact of peptides from casein and peptide-related amino acids on degranulation in rat basophilic leukemia cell line RBL-2H3. Nippon Shokuhin Kagaku Kaishi. 2012;59:556–561. (in Japanese) 10.3136/nskkk.59.556
- Ishida M, Nishi K, Watanabe H, et al. Inhibitory effect of aqueous spinach extract on degranulation of RBL-2H3 cells. Food Chem. 2013;136:322–327.10.1016/j.foodchem.2012.08.079
- Li XM, Schofield BH, Huang CK, et al. A murine model of IgE-mediated cow’s milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206–214.10.1016/S0091-6749(99)70492-6
- Horner AA, Nguyen MD, Ronaghy A, et al. DNA-based vaccination reduces the risk of lethal anaphylactic hypersensitivity in mice. J Immunol. 2005;175:8354–8364.
- Tanaka M, Nagano T, Yano H, et al. Impact of ω-5 Gliadin on wheat-dependent exercise-induced anaphylaxis in mice. Biosci Biotechnol Biochem. 2011;75:313–317.10.1271/bbb.100695
