Abstract
Technological developments in Japan based on the results of microbial research were a major pillar supporting the postwar industrial revolution. The wellspring of these advancements was the sophisticated technology used in traditional brewing, a foundation of the characteristic Japanese food culture. In this manuscript, we will describe the fermentative production of amino acids and nucleic acids following the discovery of the umami component so distinct in Japanese cuisine, which finally revealed the true power of microbial production. Thereafter, we will describe acetic acid production stemming from brewed vinegar production and the fermentative production of some other organic acids. Finally, we will delve into the massive scale of innovations achieved by the discovery of valuable micro-organisms and how they have affected the field of food.
Fermentation of amino acids
Within Japanese food culture, dashi, Japanese soup stock, plays an important role. In 1908, Dr. Kikunae Ikeda discovered that l-glutamate was the main component contributing to the umami flavor in common kombu dashi.Citation1) His discovery of the umami taste, distinct from the previously known four basic tastes, and later illumination of the substance responsible for the umami flavor are achievements worthy of special mention. The flavor enhancement business stemming from this discovery faced a rough start, but over time as the market expanded, sales increased too rapidly.
Industrial production of l-glutamate commenced in the Meiji era, but it was manufactured through protein hydrolysis. Suffering food shortages after the Pacific War, many became concerned with the use of valuable grains as a starting material and desired improvement of the nutritional state for health considerations. Thus, research aiming to utilize micro-organisms for the production of l-glutamate was pushed forward.
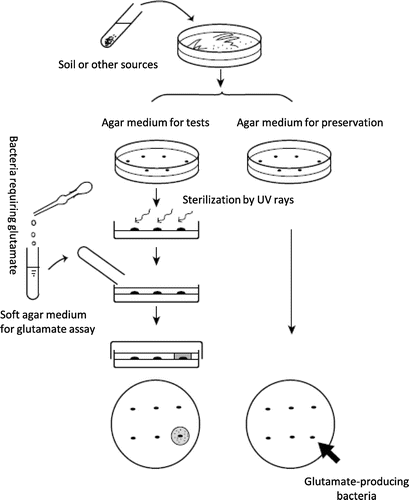
Initially, researchers tried to establish a two-step method for production: fermentative production of 2-oxoglutarate, the precursor for l-glutamate biosynthesis, and subsequent conversion of 2-oxoglutarate into l-glutamate. At that time, it might have been difficult for researchers to accept the idea that a microorganism could produce surplus extracellular l-glutamate, a substance vital to living things. In 1957, employing an elaborate, ingenious screening system and using the latest analysis technology, Dr. Shigezo Udaka and Dr. Shukuo Kinoshita of Kyowa Hakko Kogyo Co., Ltd., discovered an “inconceivable” micro-organism in the natural world (Fig. ).Citation2,3) This was the birth of amino acid fermentation.
Fig. 1. Method of screening for glutamate-producing bacteria.
Thanks to their efforts, various amino acids are now produced using micro-organisms. Mutational breeding methods were mainly used to obtain micro-organisms that produced amino acids other than glutamate: after mutagenizing a target micro-organism, mutants possessing the desired properties were selected. Analysis of the acquired mutants led to elucidation of the metabolic regulatory mechanisms and to metabolically controlled fermentation.
For glutamate fermentation, inducing cultivation conditions such as limited concentrations of biotin can lead to high glutamate yields even in wild-type strains, reaching over 20% of glucose consumed. However, for many years the underlying mechanism for excess glutamate production was cloaked in mystery and the subject of much scientific curiosity. Although the mechanism has still not been completely clarified, it is generally understood from two perspectives: (1) metabolic flux change toward glutamate biosynthesis and (2) activation of glutamate excretion into the extracellular space (Fig. ).
Fig. 2. Glutamate production mechanism in Corynebacterium glutamicum.
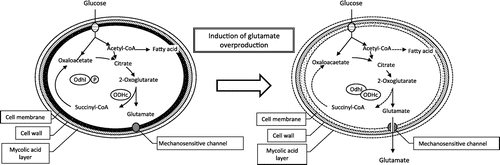
For metabolic flux change toward glutamate biosynthesis, one contributing factor is decreased activity of the 2-oxoglutarate dehydrogenase complex (ODHc), the enzyme responsible for converting the glutamate precursor 2-oxoglutarate into succinyl-CoA. Phosphorylation and dephosphorylation of the 143-aa protein OdhI have been reported to affect the regulation of ODHc activity.Citation4)
A recent study on glutamate excretion revealed that a mechanosensitive channel, which opens or closes in response to membrane tension, opens under conditions that induce glutamate production, thereby permitting glutamate to pass into the extracellular space.Citation5) These new findings are not only fascinating in regard to uncovering the mechanisms of glutamate fermentation but also have value as basic research results. They are a seed from which new applied developments are growing and will soon blossom.
Currently, the annual global demand for glutamate is estimated (2015) to be approximately 3.1 million metric tons (as a monosodium salt) and growing.
Amino acid fermentation, which was invented based on microbial screening, brought a wave of innovation to the field of biotechnology. It was not simply a fundamental technology—even today, it stands at the frontier of microbial research and contributes to enriching foods and lives worldwide.
Fermentation of nucleic acids
In 1913, Dr. Shintaro Kodama reported that the histidine salt of inosinate (IMP) was the main component of umami in katsuobushi dashi, another common Japanese soup stock.Citation6) Shortly thereafter, the contents of this report were forgotten. Nevertheless, in the late 1950s, Dr. Akira Kuninaka and colleagues discovered that 5′-IMP, an isomer of inosinate, was the substance responsible for the umami flavor.Citation7)
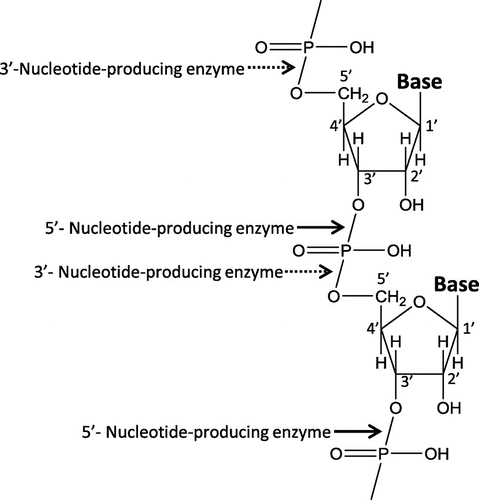
Dr. Kuninaka and his team, supported by the words of Dr. Kinichiro Sakaguchi, “It is obviously common practice in applied microbiology to ask micro-organisms to accomplish all what you desire,” continued their search to find a micro-organism capable of degrading RNA to generate nucleotides with a 5′-phosphate group; finally, they identified a fungus in the genus Penicillium that exhibited the desired activity (Fig. ).Citation8) Over the course of their research, 5′-GMP (guanylate) was also found to have an umami flavor (5′-GMP was later revealed to be the main umami component in dried shiitake mushrooms). Furthermore, when 5′-IMP and 5′-GMP were combined with monosodium glutamate, the umami flavor was synergistically enhanced. Based on these discoveries, in 1961, a compound flavor enhancer making use of the synergistic effect emerged.
Fig. 3. RNA phosphodiester bond cleavage by phosphodiesterase.
Based on the cutting-edge findings of the day on auxotrophic mutants of micro-organisms, Dr. Kuninaka and his team pursued research on the possibility of IMP production via adenine-requiring mutants. They reported their results in 1961, demonstrating that the adenine-requiring mutant of Bacillus subtilis accumulated IMP and inosine in its culture medium.Citation9) Subsequently, nucleoside and nucleotide fermentation drastically developed.
There is currently an array of methods for producing nucleotides exhibiting umami. RNA degradation, enzymatic phosphorylation of fermentation-derived inosine and guanosine, direct fermentative production of 5′-IMP, and enzymatic conversion of fermentation-derived 5′-XMP into 5′-GMP are major examples. Moreover, the industrially applied enzyme for phosphorylation of inosine and guanosineCitation10) is an example of successful enzyme improvement based on analysis of its 3D structure.
Nucleic acid fermentation, along with the amino acid fermentation mentioned above, is a representative example of successful innovation accomplished by coupling traditional Japanese food culture with advanced microbial research and applied technology. Nucleotides with umami taste have an estimated annual global demand of 31,000 metric tons (in 2011), and the market is still growing.
Fermentation of organic acids
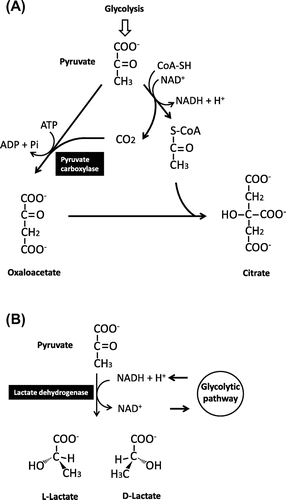
Microbial primary metabolites include amino acids, nucleic acids, and many other diverse organic acids. Numerous practical applications of fermentative production of organic acids have been achieved by utilizing the ability of a specific micro-organism to efficiently produce remarkable quantities of an organic acid. Of the organic acids generated through fermentation, citric acid in particular is produced in large quantities followed by acetic acid, lactic acid, gluconate, and itaconic acid. Today, organic acids are used for various purposes but have seen particular application as additives in the food industry where their wide-ranging effects such as adjusting sourness, extending shelf life, improving moisture retention, preventing oxidation of lipid components, accelerating solidification and coagulation, enhancing flavor, and ion chelation are highly sought.Citation11) Industrial production of organic acids has been established by discovery of micro-organisms with superior production capabilities. In this section, we will briefly discuss three organic acids (citric acid, acetic acid, and lactic acid) produced using processes based on traditional fermentation technology that enjoy expanding shares of the global market.
Citric acid
For many years, citric acid has been adopted as an additive for refreshing soft drinks and myriad other food and beverage products, and it boasts high demand worldwide. Even today, where fermentation products retain a large market, the black koji mold Aspergillus niger is still used as the primary micro-organism in mass production of citric acid.Citation12) Long ago, citric acid was produced by extraction from citrus fruits, but the first half of the twentieth century saw establishment of industrial production systems applying fermentation. For example, in 1919, the American corporation Pfizer Inc. initiated a mass-production method utilizing fermentation; in 1939, by adding sucrose as a substrate, they achieved major cost reductions for their fermentation system. This citric acid fermentation technology became an important foundation for the establishment of fermentative production of penicillin, which was also discovered around that time.
There are various suitable fermentation conditions for citric acid production, depending on the country’s economic background and supply system for raw materials, but the most common raw materials are molasses and starch. There are also many different culture methods including surface culture, liquid culture, and solid culture. For efficient citric acid fermentation, it is necessary to control specific conditions such as (1) addition of an excess carbon source, (2) retention of low pH and high dissolved oxygen content, and (3) limitations on metal ions and phosphorous.Citation13)
Citric acid is widely known for its role as an intermediate in the TCA cycle. An important step in citric acid synthesis is the ATP-dependent pyruvate carboxylase reaction that catalyzes formation of oxaloacetate by attaching a carbonate molecule to pyruvate (Fig. (A)). The definitive factors contributing to the highly efficient synthesis and excretion of citric acid by A. niger remain unclear. Based on current understanding, possible causes for excess accumulation and overflow of TCA cycle intermediates are attributed to the following three phenomena: (1) glucose uptake by the cell quickly occurs by simple diffusion; (2) glycolytic flow becomes deregulated, causing highly efficient synthesis of precursors of TCA cycle intermediates; and (3) regenerative oxidation of NADH is uncoupled, causing intracellular ATP levels and catabolic activity to remain low.Citation12) Accumulation of citric acid does not occur during vegetative growth but begins upon cessation of growth, which resembles the characteristic features of secondary metabolism.
Acetic acid
Acetic acid fermentation produces acetic acid by incomplete oxidation of ethanol. The group of bacteria possessing this ability is collectively referred to as acetic acid bacteria (AAB).Citation14) These gram-negative, aerobic bacteria are classified into the family Acetobacteraceae. The family comprises many genera, among which the genera Acetobacter and Gluconacetobacter contain bacteria exhibiting remarkable acetic acid productivity. AAB have numerous cell membrane-bound enzymes, many of which require pyrroloquinoline quinone (PQQ) and flavin-adenine dinucleotide (FAD) as coenzymes.
Acetic acid production by AAB is carried out by oxidization of the substrate through consumption of oxygen. Thus, it is differentiated from fermentation, which is narrowly defined as an energy acquisition process under anaerobic conditions, and called oxidative fermentation. During acetic acid fermentation, ethanol is oxidized into acetaldehyde by the membrane-bound alcohol dehydrogenase (ADH) with PQQ as a coenzyme. Acetaldehyde is subsequently converted into acetic acid by another membrane-bound enzyme, aldehyde dehydrogenase (ALDH). The prosthetic group of ALDH is thought to be molybdopterin-cytosine-dinucleotide. As these membrane-bound enzymes are localized on the periplasmic side of the cell membrane, acetic acid fermentation is completed outside of the cell (Fig. ). Therefore, a characteristic of acetic acid fermentation is the high yield of fermentation products obtained relative to the amount of substrate used. AAB acquire energy by transferring electrons obtained from this reaction to the electron acceptor, which is thought to be ubiquinone; however, the acceptor has yet to be identified with multiple enzymes including ALDH.
Fig. 5. Various oxidative fermentations executed by the membrane-bound enzymes in acetic acid bacteria.
Brewed vinegar has various uses in the manufacture and preparation of food products and is made from grains, fruits, or distilled alcohol as raw materials. Most vinegars are produced in aerated-agitated culture, but some use surface fermentation by static culture. In the former culture method, Acetobacter xylinus is used in the mid-acidity submerged fermentation that produces 5–10% acetic acid, whereas AAB highly resistant to acetic acid such as Gluconacetobacter europaeus are used in the high-acidity submerged fermentation with acetic acid concentrations reaching 10–20%. The latter culture method mainly involves Acetobacter pasteurianus. Among bacteria resistant to high concentrations of acetic acid, some finicky bacteria exist, for example, some do not form colonies when cultured using general microbiological methods. Furthermore, it is empirically known that AAB are genetically unstable, often undergoing phenotypic changes. One factor suggested to contribute to this instability is the existence of many mobile genetic elements in the genome such as transposons and insertion sequences.
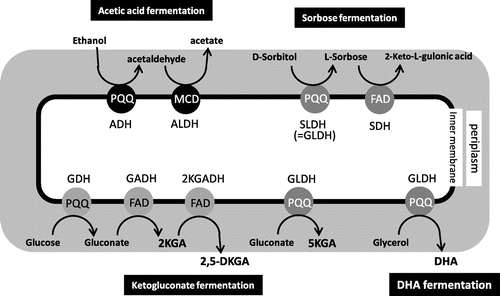
Due to their high oxidative fermentation activity, AAB have also been used in the fermentative production of some other organic acids (Fig. ).Citation15) For example, some species in the genera Gluconobacter convert d-sorbitol to l-sorbose, which is then converted to 2-keto-l-gulonic acid. The six-carbon-carboxylate 2-keto-l-gulonic acid is a direct precursor in the industrial production of vitamin C (ascorbic acid). Most bacteria in the genus Gluconobacter generate and accumulate gluconate from glucose without undergoing phosphorylation, and gluconate is further oxidized to 2-keto-d-gluconic acid (2KGA) and 5-keto-d-gluconic acid (5KGA). 5KGA is attracting much interest as a raw material for l-tartaric acid production. The reaction converting glucose to glucono-δ-lactone (lactone compound in equilibrium with gluconate) is catalyzed by membrane-bound and PQQ-dependent glucose dehydrogenase (GDH). The reactions to produce 2KGA and 5KGA are catalyzed by FAD-dependent gluconate dehydrogenase (GADH) and PQQ-dependent gluconate dehydrogenase (GLDH), respectively. GLDH is recognized as the same enzyme as sorbitol dehydrogenase (SLDH), which catalyzes conversion of sorbitol to sorbose. Furthermore, SLDH functions as a glycerol dehydrogenase, which generates dihydroxyacetone (DHA) by oxidizing glycerol.
Lactic acid
Lactic acid fermentation is a typical fermentation process in the narrow sense defined as an energy-acquisition process under anaerobic conditions.Citation11) In other words, by generating lactic acid via NADH-dependent reduction of pyruvate, the lactate dehydrogenase reaction regenerates NAD+ from the NADH formed through the oxidation reaction in glycolysis. By coupling these reactions, energy conservation and lactic acid accumulation occur during glycolysis (Fig. (B)). The main micro-organisms that perform lactic acid fermentation fall into a clade of gram-positive and low G + C content bacteria collectively referred to as lactic acid bacteria (LAB).
Using six- or five-carbon sugars, LAB perform the aforementioned metabolism; however, there are two types of fermentation: homolactic fermentation only produces lactic acid, and heterolactic fermentation produces the byproducts ethanol, carbon dioxide, and acetic acid in addition to lactic acid. The type of fermentation used is an important characteristic of each bacterium. Homolactic fermentation, in which 1 mol of a six-carbon sugar is ultimately converted into 2 mol lactic acid, is used industrially to manufacture lactic acid because of its efficiency. Heterolactic fermentation, on the other hand, infuses various flavors into its products, so it is used to manufacture fermented foods.
First established by Avery (U.S.A.) in 1881, industrialized lactic acid fermentation boasts a long history. There are a limited number of LAB species suitable for fermentative production of lactic acid. Lactobacillus delbrueckii is commonly used because it can maintain its ability to produce lactic acid even at high temperatures and can consume molasses and starch.Citation16) Rhizopus oryzae, a filamentous fungus that produces remarkable quantities of l-lactate, is also used. In lactic acid fermentation with LAB, glucose, sucrose, and starch are used as carbon sources. LAB have complex nutritional requirements, so it is necessary to add yeast extract and other nutrients to the culture medium. Thus, many types of substances are included in the culture medium, which increases the complexity and cost of the subsequent purification process required to obtain highly purified lactic acid. In contrast, for the fermentation process using the filamentous fungus R. oryzae, only starch and a tiny amount of inorganic salt are added to the culture medium, allowing relatively easy acquisition of high-purity lactic acid. However, the fungal mycelia form pellets and generate high amounts of organic acid byproducts, causing low yields compared to LAB.
After the end of WWII, compelled by soaring starch prices, chemical synthesis was applied to lactic acid production. However, fermentative production has attracted attention again in recent years because of growing demand for high-purity lactic acid in the chemical industry for production of, for example, polylactic acid, a type of biodegradable plastic. Lactic acid exists in two enantiomeric forms, l- and d-lactate (Fig. (B)). When only one of the enantiomers is desired, it is quite costly to produce through chemical synthesis. However, in lactic acid fermentation, the specificity of lactate dehydrogenase enables high-purity production of only one enantiomer. Thus, use of bacteria with high specificity allows production of highly purified lactic acid with high optical purity. According to a 2008 report, 150,000 metric tons of l-lactate are produced worldwide by fermentation.Citation17)
In addition to lactic acid, LAB have been practically used to produce a group of antimicrobial peptides called lantibiotics.Citation16) These peptides are produced by ribosome-dependent synthesis of the precursor peptides encoded by the corresponding genes followed by post-translational modification. One of the abnormal amino acids generated by such modifications, lanthionine, forms intramolecular crosslinks and is found in antibiotic substances called lantibiotics (lanthionine-containing antibiotics). Lantibiotics exhibit antimicrobial activity by damaging the membrane of bacterial cells and are effective food preservatives. In particular, nisin A, a product of Lactococcus lactis, is added as a preservative to canned foods and mayonnaise.
Concluding remarks—new fermentation technology developed through the ages
After WWII, infectious diseases and food shortages in Japan were urgent matters requiring solutions. Leveraging Japan’s specialty in fermentation, technological developments utilizing micro-organisms quickly progressed. In the wake of the discoveries of penicillin and streptomycin, the potential of research on antibiotic-producing bacteria was acknowledged. Soon thereafter, screening for such bacteria led to the discovery of a multitude of bioactive substances and the establishment of systems for their fermentative production. Meanwhile, as noted above, technology related to food additives developed similarly owing to the efforts to discover valuable microbial producers, and efficient fermentative production became a reality, propelling expansion of applications. Today, in conjunction with advances in the engineering of food product manufacturing, products with higher added value are being created, and the diversity in the roles of food additives is growing.
The pathway to obtaining valuable bacterial producers and the subsequent development of production systems using them led to advancements in research that elucidated basic metabolism and gene regulation mechanisms essential to enhanced production. Numerous findings in molecular biology thus accumulated. Currently, through metabolic engineering approaches based on these findings, breeding of bacterial producers using genetic engineering is underway in a variety of contexts. Furthermore, as seen in the examples above, valuable substances can be found in the byproducts synthesized by bacterial producers, creating additional value in the production process. Thus, one merit of fermentative production is that a single production system has is a multifaceted ripple effect spanning from applications to fundamental knowledge.
Environmental and energy problems are finally being perceived as a dire issue today, and the possibilities of fermentative production for solving the problems are attracting much attention. As demonstrated with polylactic acid, there are growing expectations for technology using micro-organisms owing to the increasing demand for new materials and the need to establish production processes based on the effective utilization of underused resources. Furthermore, climate change due to global warming and changes in logistics due to the globalization of human society could drastically affect the productivity and distribution of agricultural and fishery products, implying the need for technological developments in overcoming these issues.
“Rely on the abilities of micro-organisms and learn from them the true nature of life”—this principle, inherited by the researchers in the long-established fields of agricultural chemistry and applied microbiology, has bestowed upon us a long list of basic technologies and findings related to medicine, food, and even the environment. Through this report, we realize the necessity of continuing the pursuit of fundamental studies and technological advancements in response to modern demands with composure and flexibility.
Acknowledgment
We thank Professor Tatsuo Hoshino for his assistance in checking the description of organic acid fermentation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
† This work is a translation of an original work in Japanese in the Japan Society for Bioscience, Biotechnology, and Agrochemistry http://doi.10.1271/kagakutoseibutsu.54.54
References
- Ikeda K. J Tokyo Chem Soc. 1909;30:820.
- Kinoshita S, Udaka S, Shimono M. J Gen Appl Microbiol. 1957;3:193.10.2323/jgam.3.193
- Udaka S. J Bacteriol. 1960;79:754.
- Niebisch A, Kabus A, Schultz C, et al. J Biol Chem. 2006;281:12300.10.1074/jbc.M512515200
- Hashimoto K, Murata J, Konishi T, et al. Biosci Biotechnol Biochem. 2012;76:1422.10.1271/bbb.120366
- Shintaro K. J Tokyo Chem Soc. 1913;34:751.
- Kuninaka A. J Agr Chem Soc Jpn. 1960;34:489.10.1271/nogeikagaku1924.34.6_489
- Kuninaka A, Otsuka S, Kobayashi Y, et al. Bull Agr Chem Soc Jpn. 1959;23:239.
- Uchida K, Kuninaka A, Yoshino H, et al. Agr Biol Chem. 1961;25:804.10.1080/00021369.1961.10857882
- Ishikawa K, Mihara Y, Shimba N, et al. Protein Eng. 2002;15:539.10.1093/protein/15.7.539
- Quitmann H, Fan R, Czermak P. Adv Biochem Eng Biotechnol. 2014;143:91.
- Berovic M, Legisa M. Biotechnol Annu Rev. 2007;13:303.10.1016/S1387-2656(07)13011-8
- Papagianni M. Biotechnol Adv. 2007;25:244.10.1016/j.biotechadv.2007.01.002
- Sakusankin Kenkyukai compiled. Su no Kinou to Kagaku [Vinegar.-.its function and science] (in Japanese). Tokyo: Asakura Publishing Co. Ltd; 2012.
- Hoshino T. Microbiol Cult Collect. 2011;27:83.
- Japan Bioindustry Association. Fermentation and Metabolism Study Group compiled: Hakko handbook. Tokyo: Kyoritsu Shuppan Co Ltd; 2001.
- Sauer M, Porro D, Mattanovich D, et al. Trends Biotechnol. 2008;26:100.10.1016/j.tibtech.2007.11.006