Abstract
With the decision to award the Nobel Prize in Physiology or Medicine to Drs. S. Ōmura, W.C. Campbell, and Y. Tu, the importance and usefulness of natural drug discovery and development have been revalidated. Since the end of the twentieth century, many genome analyses of organisms have been conducted, and accordingly, numerous microbial genomes have been decoded. In particular, genomic studies of actinomycetes, micro-organisms that readily produce natural products, led to the discovery of biosynthetic gene clusters responsible for producing natural products. New explorations for natural products through a comprehensive approach combining genomic information with conventional methods show great promise for the discovery of new natural products and even systematic generation of unnaturally occurring compounds.
Introduction
Humans have a very long history of employing natural products to treat illnesses starting in 500 B.C. with the use of a plant called ipecacuanha (active ingredient: emetine) in China to treat amoebic dysentery. Later, cinchona tree bark (active ingredient: quinine) was used to treat malaria in seventeenth-century Europe. In both cases, people ingested the plant matter itself; however, H. W. Florey and E. B. Chain revolutionized medicine when they extracted and purified the antibiotic substance penicillin, originally discovered by A. Fleming in 1928, and made it available as a medicine to treat infectious diseases. Thereafter, S. A. Waksman, focusing on the soil micro-organisms actinomyces, discovered the antituberculosis agent streptomycin from Streptomyces griseus; streptomycin is still used today as a chemotherapeutic agent. For their achievements, Florey, Chain, and Fleming were awarded the Nobel Prize in Physiology or Medicine in 1945, followed by Waksman in 1952. Encouraged by Waksman’s discovery of streptomycin and actinomycin, numerous research institutes and pharmaceutical companies began conducting most of their exploratory research on actinomycete cultures, consequently, dispensing countless pharmaceuticals to the world. However, believing this kind of research to be inefficient due to the time and financial investments required, many pharmaceutical companies steadily decreased its application over the 1980s and 1990s.
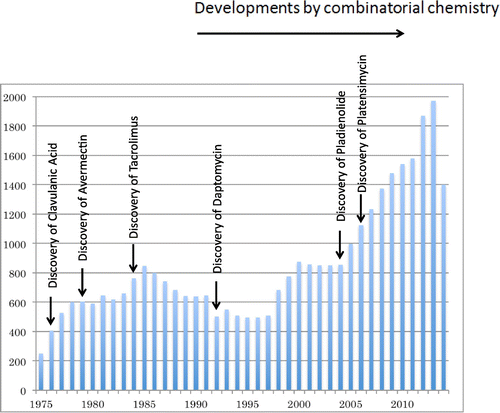
Beginning in the 1990s, combinatorial chemistry, arising from the West’s principle of efficiency, began to be used in pharmaceutical candidate discovery, and natural product discovery for pharmaceuticals was further sidelined (Table ). However, hardly any valuable pharmaceutical compounds were discovered through combinatorial chemistry. Therefore, due to the diversity of biological activity, recent years have seen a return to natural product discovery. Furthermore, the development and advancement of HPLC, mass spectrometry, and many other analytical techniques and instruments can facilitate isolation of trace amounts of substances from culture and rapid acquisition of structural information. These effects can be seen in the increasing numbers of papers published on natural products in the twenty-first century (Fig. ).
Table 1. Genome analysis of Streptomyces and related micro-organisms.
Fig. 1. Number of papers published per year on natural product isolation, taken from a literature search (SciFinder) for “Natural Product” between 1975 and 2014.
Since the end of the twentieth century, genome analyses have advanced at an accelerating pace, and the genome analyses of numerous organisms have been completed. Natural product discovery is expected to rapidly advance through the incorporation of genomic information with traditional methodologies. Because the research into the antiparasitic agent avermectin and the micro-organism that produces it, S. avermitilis—the topic that brought the Nobel Prize in Physiology or Medicine to Dr. Ōmura—is expected to make a substantial contribution to future natural product research in the post-genome era, I would like to discuss the course and results of Dr. Ōmura’s research and touch on the future prospects of natural product research.
Genome analysis of actinomycetes
Since genome analysis of numerous organisms began at the end of the twentieth century, over 7000 complied micro-organism genomes have been reported (https://gold.jgi.doe.gov/). Among micro-organisms, actinomycetes, cyanobacteria, and myxobacteria are known as prominent producers of natural products. In particular, many metabolites from actinomycetes are currently used as pharmaceutical drugs. The Streptomyces genome sequence was published at the start of this centuryCitation1,2); in 2001, a draft genome of S. avermitilis—producer of avermectin and an antiparasitic agent predominately used industrially—was published, and in 2003, its complete genome was published.Citation3) In the following years, genomes from 19 strains of Streptomyces, including S. avermitilis, have been deposited into the U.S. NCBI database (Table ).
Typically, the chromosome of prokaryotic micro-organisms consists of double-stranded, circular DNA, but the deposited Streptomyces strains did not have a circular chromosome; in fact, they all possessed a linear chromosome constituted from double-stranded DNA just like eukaryotic organisms. Furthermore, the chromosome has long terminal-inverted repeats in the telomeric region of both ends, and the 5′-end is covalently linked to the basic protein Tpg through a phosphoester bond to a Ser residue, as observed on the ends of Bacillus subtilis phage ϕ29 and adenovirus genomes. We identified an origin of replication (oriC) with at least 19 DnaA box-like sequences, almost the same as that of circular bacterial chromosomes. Although replication proceeds in both directions from the origin of replication, the terminal region of the lagging strand cannot undergo replication, suggesting that a terminal protein primes the terminal region of the chromosome. Currently, outside of Streptomyces, Rhodococcus jostii RHA1Citation4) and Kitasatospora setae KM-6054Citation5) are the only other actinomycetes that possess a linear chromosome. In addition, plasmids with either circular or linear structures exist in a few Streptomyces species.
The chromosome of Streptomyces is rather large for a bacterium, 6.84–12.70 Mbp, with 70–73% GC content and 5800–10,000 protein-coding genes, more than that found in the lower eukaryotes Saccharomyces cerevisiae (6294 genes) and Schizosaccharomyces pombe (4824 genes). Recently, the genome sequences of Streptomyces and other closely related species of bacteria (erythromycin-producing Saccharopolyspora erythraea NRRL 2338Citation6) and vancomycin-producing Amycolatopsis orientalis HCCB10007Citation7)) were announced with sizes and numbers of genes similar to Streptomyces (Table ). The gene products encoded by Streptomyces and related species—besides the obvious bioactive substances including gene clusters for secondary metabolite biosynthesis—included many duplicate genes (about 30% of the genes were identical duplicated paralogs) and genes involved in regulation (about 10%). Generally, there is a correlation between genome enlargement and an increase in regulatory genes.Citation8) Given the soil environment in which these fungi exist, the myriad regulatory systems are thought necessary to allow quick adaptation to diverse environments.
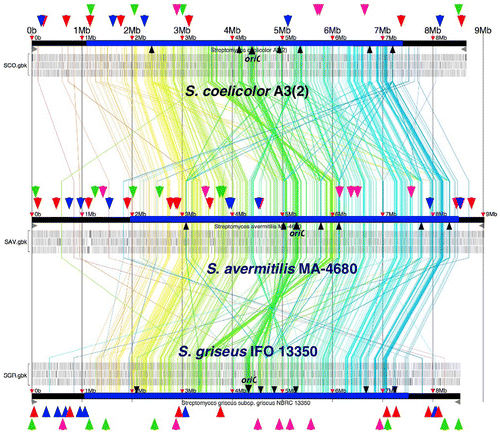
Many differences can be discerned from the dissimilarity of the Streptomyces linear chromosome and the usual circular bacterial chromosome. Genes essential for growth, such as those for replication, transcription, translation, and other primary metabolites, form a 6-Mbp region centered at the origin of replication (3 Mbp in each direction) near the middle of the linear chromosome. Strain-specific genes were located in a 1–1.5-Mbp subtelomeric region on each end, totaling 2–3 Mbp, in addition to many gene clusters for secondary metabolite biosynthesis (Fig. ). Furthermore, as many genes suspected to be transposable elements or insertion sequences were also located in the subtelomeric regions, it is thought that a large number of horizontal gene transfers have occurred in this region. Streptomyces strains possess many duplicate genes, as seen in a typical bacteria genome, but lack resolvase genes (xerC and xerD) for separating the dimeric chromosomes (concatemers) at the final stage of replication, likely because their function became obsolete for linear chromosomes, leading to their eventual loss. Bacteria are equipped with a homologous recombination mechanism for repairing damaged DNA and multiple recombination mechanisms exist. In Escherichia coli, the recB-recC-recD system plays an important role in recombination, but recB and recC are missing in Streptomyces. Most likely, recombination repair via the recF gene product could be the main pathway. Genes encoding cytochrome P450, which catalyzes the oxygenation reaction using molecular oxygen, can be found in 20–30 loci throughout the genome. Many other genes for the electron partner of the oxygenation reaction, ferredoxin, and the corresponding reduction enzyme can also be found. Another distinctive feature of the genome is that it possesses many RNA polymerase sigma factor (~60) and Ser/Thr protein kinase (20–30) genes—which might be connected to the large number of regulatory genes.
Fig. 2. Comparative analysis of chromosome structures in the genus Streptomyces and distribution of secondary metabolite biosynthesis genes (cluster).
Discovery of gene clusters for secondary metabolite biosynthesis by genome mining
The major interest in Streptomyces is the wide variety of secondary metabolite structures and bioactivities that are produced by Streptomyces species. Furthermore, these species have the metabolic capacity to supply precursors for industrial-level production of those secondary metabolites, which makes it easy to understand why these organisms play an essential role in the production of substances. Although gram-negative bacteria (cyanobacteria and myxobacteria) also produce relatively large quantities of secondary metabolites, they lack the capacity of Streptomyces. Before the Streptomyces genome sequence was published, it was thought that only a few types of biosynthetic gene clusters for secondary metabolites, including major metabolites, existed in micro-organisms that produce secondary metabolites. However, upon decoding the genomes of S. avermitilis and S. coelicolor A3(2), the number of biosynthetic gene clusters discovered (20–37) far exceeded expectations. Thereafter, the genomes of the streptomycin-producing micro-organism, S. griseus, and many other species of Streptomyces were decoded, reconfirming the earlier findings. In total, the biosynthetic gene clusters constitute more than 5–7% of the chromosome. Thus, one reason that secondary metabolites, including many antibacterial substances, are produced by Streptomyces strains is that a single strain possesses a diversity of gene clusters for secondary metabolite biosynthesis. However, not all of the putative biosynthetic gene clusters in the genome are expressed; instead, many gene clusters remain silent.
When the genome analysis of S. avermitilis was being conducted, the public data available on protein functions and annotated proteins were not nearly as complete as it is today. Hence, while half of the gene products were recognized as homologous with the public data, we could not predict their functions because they were “hypothetical proteins.” At that time, information on elucidated biosynthetic genes for secondary metabolite was scarce, and much verification was necessary to predict gene clusters for secondary metabolite biosynthesis. Now, the public database is well furnished thanks to the genomic analyses of numerous species, and gene functions can be deduced to an extent using BLAST and FASTA. In addition to function prediction by homology, protein family (Pfam) analysis using statistical models (hidden Markov model; HMM) has become common, and the statistical model AnitSmash,Citation9) which specializes in gene products of secondary metabolite biosynthesis, was made public.
Current methods allow very accurate protein function predictions, and evaluation of biosynthetic gene clusters is better than ever before thanks to the markedly improved processing capabilities of computers. Accordingly, the methodology for determining genes encoding secondary metabolite biosynthesis from genome sequence data is almost complete. In recent years, with increased use of next-generation sequencing, both the processing speed and accuracy of DNA nucleotide sequence analyses have improved, decreasing the cost 500-fold relative to that when S. avermitilis was sequenced. Nowadays, “one laboratory, one genome sequencing” has become reality.
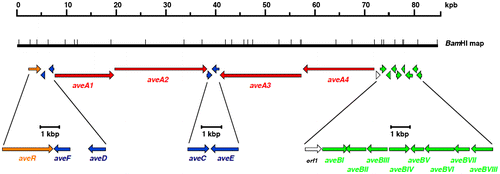
The biosynthetic analysisCitation10–12) of avermectin in S. avermitilis is a prominent example of biosynthetic gene cluster research on secondary metabolites up until the end of the twentieth century. By mutagenesis of S. avermitilis, we obtained various types of biosynthetically blocked mutants. Classification of the mutant strains was carried out along with isolation and structural analysis of the accumulated intermediates to discern the biosynthetic pathways. For an even more detailed analysis, the biosynthetic gene cluster for avermectin was cloned. At that time, information on nucleotide sequences of biosynthetic genes, which is available today, was limited, so one could not simply obtain a clone containing the desired gene fragment by hybridization, PCR, or other methods using homology with the nucleotide sequence. Therefore, we constructed chromosomal DNA libraries from the wild-type strain and introduced these into biosynthetically blocked mutants as hosts. We selected clones that were complementary to the host’s mutated genotype obtained from the library, and we deduced a part of the biosynthesis gene cluster. To obtain the entire biosynthetic gene cluster, we used the DNA fragments that contained a part of the acquired biosynthesis gene cluster, hybridized the constructed wild-type strain library and the colonies using a predefined cosmid vector, and then selected the cosmid clones that had incorporated the upstream and downstream regions of the cloned fragment. Through this method, we covered the entire gene cluster for avermectin biosynthesis with four cosmid clones. The final step, manually decoding the sequence of these cosmid clones by the dideoxy method using polyacrylamide gel electrophoresis, took over two years, but we were able to decipher the complete, 85-kbp biosynthetic gene cluster for avermectin (Fig. ).Citation11,12)
Fig. 3. Physical map of avermectin biosynthesis gene clusters: regulatory genes (orange), polyketide synthase genes (red), polyketide modification enzyme genes (blue), and glycosidation and sugar biosynthesis genes (green).
Nowadays, isolation of biosynthetically blocked mutants using the above method is not a part of researching the biosynthesis of metabolites of interest; one simply decodes the genome sequence of micro-oraganisms. There is information on many biosynthetic genes for secondary metabolites listed in the public database, and careful comparison of obtained sequence data with the database enables the use of bioinformatics to analyze homology and protein families and discover the region encoding biosynthetic genes of interest. In the end, we introduced the clone containing all of the biosynthetic genes to a heterologous host to check whether the target metabolite was produced or if we obtained a strain with blocked biosynthesis and whether the mutated genotype from the introduced cloned fragment was complementary. However, the biosynthetic genes for polyketide compounds produced by type-I polyketide synthase, such as avermectin, erythromycin, and rapamycin, include a multifunctional enzyme gene much larger than can be stored on a cosmid vector. Consequently, we could not obtain a cosmid clone incorporating the entire biosynthetic gene cluster. In such situations, the region involving biosynthesis of secondary metabolite on the chromosome of the producer was deleted via homologous recombination using cloned fragments to obtain a recombinant strain. If the region deleted from the chromosome was the targeted biosynthetic gene(s), production of the metabolite would cease, or the intermediate(s) would be accumulated. Thus, until the end of the twentieth century, advancing to the analysis of genes involved in biosynthesis when conducting research on secondary metabolite biosynthesis required a very long time. However, with the technological innovations of recent years (separation techniques, mass spectrometry, sequencing, etc.), it is now possible to decipher gross secondary metabolite biosynthesis in a very short time.
Heterologous gene expression system for secondary metabolite production
As mentioned above, genome analyses of S. avermitilis and S. coelicolor A3(2) revealed numbers of biosynthetic gene clusters in the genus Streptomyces greatly exceeding our expectations. However, most of these were silent. Bacterial growth does not necessarily coincide with secondary metabolite production. If some metabolites are produced in a variety of growth conditions, then there must also be metabolites that are only produced under special growth conditions. S. avermitilis only produces avermectin in a high-glucose content medium under conditions with slightly less shaking; otherwise, if medium or culture conditions are altered, only the type-I polyketide compounds, oligomycin and filipin, were accumulated but not avermectin. In particular, scaling-up the culture is complicated, and avermectin is not produced in a typical jar fermenter. Large quantities of avermectin cannot accumulate unless the oxygen content is raised to a certain level at the start of the fermentation and agitation is decreased after production initiates. In this case, the biosynthesis gene cluster is “silent” because its transcription is not verified when avermectin is not being produced. However, when grown in high-glucose content medium, the biosynthetic gene cluster is “active” because accumulation of avermectin and transcription of the biosynthesis genes are verified.
There are at least 37 biosynthetic gene clusters for secondary metabolites in the S. avermitilis genome, 15 of which have been verified to produce metabolites based on their accumulation.Citation13) As a few of those are not expressed under normal conditions, we utilized a forced expression system. The same was observed for S. coelicolor A3(2) and the streptomycin-producing S. griseus; under appropriate conditions, both expressed 25–53% of their biosynthetic gene clusters, and the products were observed.Citation13) The “silent state” does not indicate gene loss of function through mutation, insertion, or deletion; it was realized that the transcription of all of the biosynthetic genes is not linked, so some products will not be attained. The only known example of a mutation, insertion, or deletion of a biosynthetic enzyme gene in S. avermitilis is the terpene synthase gene that helps make 2-methylisoborneol (produced by actinomycetes, cyanobacteria, myxobacteria, or the filamentous fungi Penicillium). It is thought that insertion of ISSav4C at 1.246 Mbp upstream of the S. avermitilis terpene synthase gene led to deletion of the C-terminus of the downstream terpene synthase and complete loss of function.
In recent years, revolutionary advances in genome sequencing technology have facilitated sequence data acquisition for analyses of biosynthetic gene clusters for secondary metabolites. However, various problems arise when analyzing sequences concerning biosynthetic gene clusters from Streptomyces. For example, when we stored producing strains for a long time, the productivity was decreased or even completely failed. We selected clones displaying comparatively good production quantity by single-spore isolation. Even with those manipulations, however, production would halt, or the capacity would become extremely reduced. In further trials, we acquired a cosmid clone containing the entire biosynthetic gene cluster, but introducing it to the original strain was exceedingly difficult. For such cases, a system allowing incorporation of the DNA fragment containing the entire biosynthetic gene cluster into an appropriate host known for comparatively simple genetic manipulation before evaluating the metabolites would be valuable. Furthermore, if heterologous expression could be carried out in a host that can evaluate the aforementioned “silent genes” as well as apply a forced expression system, then detailed analysis would be possible. Thus, whether we can use the strain as a host for heterologous expression for this kind of production is of paramount importance. The major interest in Streptomyces is the diversity of secondary metabolites produced; however, another important characteristic is that its metabolic system (primary metabolism) supplies precursors, energy, and coenzymes that can securely produce the desired metabolites on an industrial scale. Taking great interest in this, and not satisfied with only succeeding in the laboratory, we aimed to construct a new production system for industrial-level applications.
S. avermitilis is the innate supply source for the antiparasitic agent, avermectin, and the metabolic regulation system that starts with the supply of precursors involved in the biosynthesis of secondary metabolites is thought to be well suited for industrial-scale production. In addition to producing substances of industrial worth, a quintessential quality of industrial micro-organisms is the stability of various genotypes. Furthermore, when a new biosynthetic system is constructed in a host cell by introducing a heterologous biosynthetic gene cluster, it is desirable to simultaneously stop generation of the host’s endogenous metabolites. Otherwise, the target metabolite must be separated from the endogenous metabolite after fermentation completes; or, if the substance is synthesized using a shared precursor, they will compete with each other, leading to lower production or even total loss of one of the metabolites. In the course of research on transposon mutagenesisCitation14) in S. avermitilis, we found that production of the byproduct “oligomycin” was stopped by inserting a transposon into the biosynthetic gene cluster for oligomycin, which increased avermectin production. Conversely, transposon mutagenesis in the biosynthetic gene cluster for avermectin increased production of oligomycin. From the culture media of a double deletion mutant strain lacking the biosynthetic gene clusters for both avermectin and oligomycin, we detected the previously undetected sesquiterpene antibacterial neopentalenoketolactone.Citation15) It is believed that although the biosynthesis gene cluster is being expressed, the majority of a shared precursor is being used in production of other secondary metabolites. Thus, it is important to first delete the biosynthetic gene clusters of the main endogenous products in a host so they can be used in heterologous expression.
In the genus Streptomyces, S. avermitilis is one of the few bacteria in which various genetic manipulations, such as DNA transformation, transposon mutagenesis, and homologous recombination, are well optimized. Using comparative analysis of the genomes from S. avermitilis and other Streptomyces, we found that a 6–6.5-Mbp region, including oriC at the center of the chromosome, is shared among species. We constructed S. avermitilis SUKA17 (7.35 Mbp), a strain in which 20% of the wild-type genome was deleted by homologous and site-specific recombination.Citation16) To maintain the supply of precursors used in the production of the secondary metabolite, genes involved in primary metabolism were kept, and the left subtelomeric region containing the biosynthesis gene clusters of main products was deleted to minimize genome size. The large deletion strain SUKA17 displayed no changes in the formation of aerial hypha and spores; rather, spore formation was invigorated. We observed no difference for cultures on solid medium, but the growth rate in liquid medium slightly increased while the quantity of bacteria during the stationary phase almost doubled compared to the wild-type strain. Because genes involved in primary metabolism were retained, the strains could be grown in minimum medium consisting of glucose, ammonium sulfate, and inorganic salts. Moreover, because the biosynthetic gene clusters of major metabolites like avermectin were deleted, production of secondary metabolites could not be verified either in the cultured broth or inside the mycelium cultured from various media.
With high feasibility for the prominent metabolic ability of S. avermitilis in industrial production of avermectin, we tested heterologous expression of various biosynthetic gene clusters for secondary metabolites using S. avermitilis SUKA17 (SUKA22 was predominately used later). To date, we have introduced over 30 types of heterologous biosynthetic gene clusters and verified the synthesized metabolites through expression of biosynthetic gene clusters.Citation16–18) Furthermore, when heterologous expression was recognized, it was clear that the S. avermitilis deletion mutants had better production than the original strain (Fig. ). The original strain had to produce both the major metabolites and the heterologous secondary metabolites; thus, primary metabolism had to supply precursors for both. Conversely, major metabolite production was halted in deletion mutants, so it is thought that primary metabolism could efficiently supply biosynthetic precursors for only the newly introduced heterologous secondary metabolism.
Fig. 4. Heterologous expression of gene clusters for secondary metabolite biosynthesis in an engineered S. avermitilis strain.
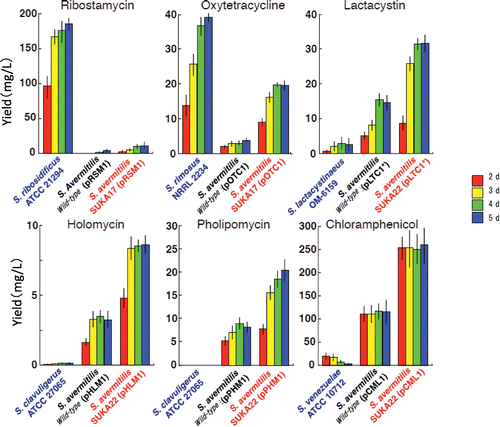
For most of these heterologous biosynthetic gene clusters for secondary metabolites, target metabolite production could be verified by simply inserting the DNA fragment containing the biosynthetic gene cluster, but a handful of biosynthetic gene clusters required forced expression of regulatory genes or direct forced expression of the biosynthetic gene clusters. For example, even upon inserting a BAC clone (75 kbp) containing the entire gene cluster for pladienolide biosynthesis (an antitumor type-I polyketide compound) into S. avermitilis SUKA17, pladienolide was not produced. Transcription analysis revealed that none of the genes in the biosynthetic gene cluster and the regulatory gene controls the expression of biosynthetic genes. It was thought that the regulatory system or factor that can activate the transcription of the regulatory gene was lacking in S. avermitilis, so we linked the transcribable promoters to start transcription in S. avermitilis and kept the regulatory gene separate, thereby accumulating a remarkable quantity of pladienolide.Citation16) In addition, the proteasome-specific inhibitor lactacystin, which performs various functions within the cell, such as cell cycle regulation, immune response, and signal transduction, is synthesized by five biosynthetic genes. As observed for the pladienolide biosynthesis gene cluster, introduction of the lactacystin biosynthesis gene cluster into the SUKA17 strain did not produce lactacystin. As the biosynthetic gene cluster for lactacystin lacks regulatory gene(s), we thought that transcription would be initiated for the biosynthetic gene cluster by a special transcription activator or factor that does not exist in S. avermitilis. By placing a promoter anticipated to facilitate good transcription in S. avermitilis upstream of the first gene of the cluster, we achieved a significant amount of lactacystin because all genes are a unidirectional transcription unit.Citation17)
The industrial micro-organism S. clavuligerus ATCC 27064, which predominately produces the important antibacterial drugs cephamycin C and clavulanic acid, did not produce other compounds. Through genome mining, we discovered the biosynthetic gene cluster for a glycolipid compound, but its production has not been verified under any culture conditions. Therefore, the biosynthetic gene cluster is silent in S. clavuligerus ATCC 27064. However, a large quantity of pholipomycin was produced by the large deletion host S. avermitilis SUKA17 (Fig. ). The biosynthetic gene cluster for pholipomycin was silent in S. clavuligerus ATCC 27064, but S. avermitilis is thought to possess a factor for activating expression of the cluster and inducing biosynthesis of pholipomycin. To the best of our knowledge, this is the sole example of enabling production by introducing a silent biosynthetic gene cluster into a host capable of expressing it.Citation17) Many of gene clusters for the secondary metabolite biosynthesis in a micro-organism genome are silent; however, if silent biosynthetic genes (clusters) can be activated like the pholipomycin biosynthetic gene cluster, then we can expect more studies of compounds that previously could not be evaluated.
Synthesis of novel secondary metabolites by forced expression of silent genes
Actinomycetes produce natural products with diverse structures and biological activities such as streptomycin, erythromycin, and FK-506. Production of secondary metabolites from filamentous fungi and plants is also well known. Of these, among terpenoid metabolites in particular, tens of thousands of metabolites including essential oils have been reported, leading terpenes to be known as the typical metabolites in plants. Terpene compounds produced by actinomycetes have not been studied to the extent of those from fungi or plants, and while production of the odoriferous substances geosmin and 2-methylisoborneol is widely known, they are considered rare metabolites. Research on terpene synthases (a group of enzymes that catalyze the production of a circularized compound by dephosphorylations of acyclic prenyl diphosphate) from bacteria, including actinomycetes, has not advanced as far as plant synthase research, and isolation of sesquiterpene synthase from Streptomyces in 1984 is the sole reported case.Citation12) Plant terpene synthases bear 252 amino acid residues at N-terminal domain that form an α-barrel structure. Because distinct motif sequences have been discovered in various plant-origin terpene synthases, a BLAST homology search for this region can be done. In contrast, terpene synthases from bacteria such as Streptomyces lack such distinct sequences; furthermore, the entire sequence has extremely low sequence homology with plant synthases, which makes gene cloning by homology very complicated. This has been a tremendous obstacle in the advancement of bacterial terpene research.
In 2008, we discovered the first-ever genes encoding 2-methylisoborneol biosynthesis with a method based on the hidden Markov model (HMM) obtained from the metal ion binding motif in the terpene synthase active site.Citation19) Then, after the HMM was further improved by presumptive bacterial terpene synthases, we predicted 262 proteins to be terpene synthases from a public database of 8,759,463 bacterial proteins.Citation20) The predicted terpene synthases were predominately found from actinomycetes, but we surmised that a proportionally large amount of data from those bacteria was deposited in the public database. In addition, it was of great interest that we found candidate synthases in gram-negative bacteria from the orders Myxococcales, Oscillatoriales, Nostocales, Burkholderiales, Herpetosiphonales, Rhizobiales, Chlamydiales, Flavobacteriales, Chromatiales, Ktedonobacterales, Sphingobacteriales, and Pseudomonadales. Furthermore, there were no predicted terpene synthases found in the gram-positive phylum Firmicutes or archaebacteria. Production of terpenoid compounds other than the odorous substances mentioned above has been scarcely reported; therefore, we concluded that the respective synthase genes were silent in the strains from which they were detected.
Typically, in studies of terpene synthases, the recombinant synthases were prepared in E. coli. Following acquisition of the recombinant protein, with the presence of Mg2+ and the substrate (geranyl diphosphate (GPP), farnesyl diphosphate (FPP), or geranylgeranyl diphosphate (GGPP)) are used for the cyclization reaction. However, the expression of actinomycete terpene synthases in E. coli frequently resulted in the formation of inclusion bodies preventing measurement of enzyme activity. Of the four genes encoding terpene synthases present in the S. avermitilis genome, only one could be expressed in E. coli as a soluble protein; the remaining three were collected as inclusion bodies. The S. avermitilis deletion mutants discussed earlier lost not only the biosynthetic gene clusters of major metabolites but also three of the four terpene synthase genes (the remaining gene was silent under all conditions, not deleted). Therefore, cyclic terpene compounds are not generated under any culture conditions in the large deletion strains SUKA17 and SUKA22.
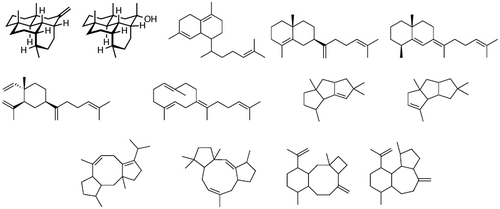
Terpene compounds generate various types of terpene hydrocarbons and alcohols through a cyclization reaction by terpene synthase using the precursor GPP, FPP, or GGPP. The Streptomyces genome contains numerous paralogs of the FPP synthase gene, but retention of GPP and GGPP synthase genes is unique to each strain. With high feasibility for a supply of efficient precursors, we positioned the GPP, FPP, and GGPP synthase genes upstream of a highly expressed promoter in S. avermitilis; while downstream of those synthase genes, a terpene synthase gene was placed to form an operon. Finally, a vector was constructed that could be stably integrated into the specific site of Streptomyces chromosome, and the newly discovered synthase genes were evaluated. Our research found that many genes generated terpenes usually produced by plants, and we have discovered 13 previously unidentified terpenes with novel structures.Citation21) In the past, terpenes were considered rare metabolites in actinomycetes; however, through this research, it was concluded that the terpene synthase genes are predominately silent and are widely distributed in bacteria.
Concluding remarks
The antituberculosis agent streptomycin discovered during the mid-twentieth century by S. A. Waksman spurred numerous researchers to explore actinomycete secondary metabolites for valuable pharmaceuticals. Beginning at the end of the twentieth century, the actinomycete genome was sequenced. We learned that there are more biosynthetic gene clusters for secondary metabolites in the micro-organism genome than we had imagined, and it was revealed that most of those genes are silent. Therefore, only a fraction of the abilities of micro-organisms was being exploited. After the turn of the century, we saw the advancement of numerous molecular genetics methods, and heterologous expression systems of biosynthetic gene clusters became practical in actinomycetes. Metabolites unobtainable with past methodologies were now within reach using new technologies to awaken silent gene clusters. Actinomycete genome analysis triggered research on the expression system of heterologous biosynthetic gene clusters. Analysis of the avermectin-producing S. avermitilis genome started in 2000 under the leadership of Prof. Ōmura and was completed in 2003. Initially, we did not imagine our research would lead to heterologous expression systems of biosynthetic gene clusters for secondary metabolites; however, many research developments became promising thanks to bacterial genome sequencing. In 1985, through collaboratinon with D. A. Hopwood, we reported the creation of a novel hybrid antibiotic by genetic manipulation.Citation22) Although this method depended on the capabilities of the producing micro-organism, a complete heterologous production system allows generation of a hybrid metabolite independent of the producing micro-organism capabilities. It is thought that this point will be reached soon.
Disclosure statement
No potential conflict of interest was reported by the author.
Acknowledgments
The series of research accomplishments chronicled in this manuscript were achieved thanks to Prof. Ōmura’s support and valuable advice concerning avermectin biosynthesis research and more. Furthermore, I admire the foresight of Prof. Ōmura, who gave me the extremely valuable and exciting challenge, at the time, to analyze the S. avermitilis genome. The S. avermitilis genome analysis was the starting point of the conception of constructing heterologous expression systems for gene clusters for secondary metabolite biosynthesis. Along with congratulating Prof. Ōmura on his Nobel Prize in Physiology or Medicine, I would like to offer my sincere gratitude.
Notes
† This work is a translation of an original work in Japanese in the Japan Society for Bioscience, Biotechnology, and Agrochemistry http://doi.10.1271/kagakutoseibutsu.54.17
References
- Ōmura S, Ikeda H, Ishikawa J, et al. Genome sequence of an industrial microorganism Streptomyces avermitilis: Deducing the ability of producing secondary metabolites. Proc Natl Acad Sci USA. 2001;98:12215–12220.10.1073/pnas.211433198
- Bentley SD, Chater KF, Cerdeño-Tárraga A-M, et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature. 2002;417:141–147.10.1038/417141a
- Ikeda H, Ishikawa J, Hanamoto A, et al. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol. 2003;21:526–531.10.1038/nbt820
- McLeod MP, Warren RL, Hsiao WW, et al. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc Natl Acad Sci USA. 2006;103:15582–15587.10.1073/pnas.0607048103
- Ichikawa N, Oguchi A, Ikeda H, et al. Genome sequence of Kitasatospora setae NBRC 14216T: An evolutionary snapshot of the family Streptomycetaceae. DNA Res. 2010;17:393–406.10.1093/dnares/dsq026
- Oliynyk M, Samborskyy M, Lester JB, et al. Complete genome sequence of the erythromycin-producing bacterium Saccharopolysporaerythraea NRRL23338. Nat Biotechnol. 2007;25:447–453.10.1038/nbt1297
- Tang B, Wang Q, Yang M, et al. ContigScape: a Cytoscape plugin facilitating microbial genome gap closing. BMC Genomics. 2013;14:289.10.1186/1471-2164-14-289
- Stover CK, Pham XQ, Erwin AL, et al. Complete genome sequence of Pseudomonasaeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964.
- Weber T, Blin K, Duddela S, et al. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43:W237–243.10.1093/nar/gkv437
- Ikeda H, Kotaki H, Omura S. Genetic studies of avermectin biosynthesis in Streptomyces avermitilis. J Bacteriol. 1987;169:5615–5621.
- Ikeda H, Nonomiya T, Usami M, et al. Organization of the biosynthetic gene cluster for the polyketide anthelmintic macrolide avermectin in Streptomycesavermitilis. Proc Natl Acad Sci USA. 1999;96:9509–9514.10.1073/pnas.96.17.9509
- Ikeda H, Nonomiya T, Omura S. Organization of biosynthetic gene cluster for avermectin in Streptomyces avermitilis: Analysis of enzymatic domains in four polyketide synthases. J Ind Microbiol Biotechnol. 2001;27:170–176.10.1038/sj.jim.7000092
- Nett M, Ikeda H, Moore BS. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat Prod Rep. 2009;26:1362–1384.10.1039/b817069j
- Ikeda H, Takada Y, Pang CH. Transposon mutagenesis by Tn4560 and applications with avermectin-producing Streptomyces avermitilis. J Bacteriol. 1993;175:2077–2082.
- Tetzlaff CN, You Z, Cane DE, et al. A gene cluster for biosynthesis of the sesquiterpenoid antibiotic pentalenolactone in Streptomyces avermitilis. Biochemistry. 2006;45:6179–6186.10.1021/bi060419n
- Komatsu M, Uchiyama T, Omura S, et al. Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc Natl Acad Sci USA. 2010;107:2646–2651.10.1073/pnas.0914833107
- Komatsu M, Komatsu K, Koiwai H, et al. Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synth Biol. 2013;2:384–396.10.1021/sb3001003
- Ikeda H, Shin-ya K, Omura S. Genome mining of the Streptomyces avermitilis genome and development of genome-minimized hosts for heterologous expression of biosynthetic gene clusters. J Ind Microbiol Biotechnol. 2014;41:233–250.10.1007/s10295-013-1327-x
- Komatsu M, Tsuda M, Omura S, et al. Identification and functional analysis of genes controlling biosynthesis of 2-methylisoborneol. Proc Natl Acad Sci USA. 2008;105:7422–7427.10.1073/pnas.0802312105
- Yamada Y, Kuzuyama T, Komatsu M, et al. Terpene synthases are widely distributed in bacteria. Proc Natl Acad Sci USA. 2015;112:857–862.10.1073/pnas.1422108112
- Yamada Y, Arima S, Nagamitsu T, et al. Novel terpenes generated by heterologous expression of bacterial terpene synthase genes in an engineered Streptomyces host. J Antibiot (Tokyo). 2015;68:385–394.10.1038/ja.2014.171
- Hopwood DA, Malpartida F, Kieser HM, et al. Production of ‘hybrid’ antibiotics by genetic engineering. Nature. 1985;314:642–644.10.1038/314642a0