Abstract
To actualize the invention of all-Japanese medicines, the Department of Innovative Drug Discovery and Development (iD3) in the Japan Agency for Medical Research and Development (AMED) serves as the headquarters for the Drug Discovery Support Network. iD3 assists with creating research strategies for the seeds of medicines discovered by academia and provides technological support, intellectual property management, and aid for applying the seeds through industry-led efforts. In this review, from the perspective of a science coordinator, I will describe the current activities of the drug discovery support network and iD3 as well as the challenges and future developments of pharmaceutical research and development using the natural product drug discovery method.
I would like to congratulate Dr. Satoshi Ōmura, Distinguished Emeritus Professor of Kitasato University, on receiving the Nobel Prize in Physiology or Medicine.
The enormous value of the achievements of drug discovery research from natural products conducted at Japanese universities and research institutions has finally been acknowledged. Long awaited by everyone involved, Prof. Ōmura’s Nobel Prize was fantastic news and bestows immense hope and encouragement to all—an accomplishment truly worth commemorating. Through this, I hope that interest in natural product drug discovery grows, more drugs are discovered that contribute to human health like avermectin, and research and development activities in this field take off.
When news of the award was released, a wave of excitement spread across Japan praising Prof. Ōmura. Though delightful, there are difficulties surrounding natural product drug discovery in Japan. Until a few years ago, presentations of compounds linked to drug discovery seeds were often seen at annual conferences of The Japan Society for Bioscience, Biotechnology, and Agrochemistry, but recently the number has considerably dwindled. I worked in the field of microbial natural product drug discovery for almost 30 years at Fujisawa Pharmaceutical Co., Ltd. and Astellas Pharma Inc., and I find it very unfortunate that Japanese pharmaceutical companies are increasingly distancing themselves from natural product drug discovery research.
Does this mean that there is no future for innovative natural product drug discovery research? The answer is “NO.” The readers of this journal would certainly not deny that nature is still a valuable treasure chest of new discoveries for humanity. Considering that currently less than 10% of microorganisms in the soil are being used, microbial resources remain a very appealing resource for drug discovery screening. In addition, if advancements in scientific technology bring the development of new microorganism culture methods, the discovery of new drug targets, and the development of novel assay methods, we can expect continuous emergence of new pharmaceuticals from natural product drug discovery.
In this manuscript, I will introduce iD3, which mainly strives to support practical applications of innovative seeds for drug discovery from Japanese academia. Thereafter, I will discuss the challenges faced by the practical application of microbial drug discovery seed compounds and the future developments of innovative natural products drug discovery research.
iD3 and the Drug Discovery Support Network
I am serving as a science coordinator at the East Japan Office iD3, AMED, which was established in April 2015. AMED supports research and development, leaving no gaps on the path from basic research to practical applications and implementing integrated research management. We promote various projects to reach our goal of providing the world’s highest level of medical care and services and contributing to the formation of a “society of health and longevity.” iD3 is one of six operating departments in AMED and is located at the West Japan Office and East Japan Office in Osaka and Tokyo, respectively. AMED’s mission is to support research and development of medical fields that can actualize the three LIFEs—seimei, seikatsu, and jinsei (three Japanese words that all mean “life” but also roughly translate to existence, livelihood, and lifetime). Furthermore, iD3 aims to contribute to the acquisition of new therapeutic drugs that will lead to realization of the three LIFEs by comprehensively supporting scientifically highly suitable drug discovery seeds.
Originally, in 2013, iD3 was established as the Office of Innovative Drug Discovery and Development in the National Institute of Biomedical Innovation. The “Drug Discovery Support Network” was formed by connecting the National Institute of Biomedical Innovation, RIKEN, and the National Institute of Advanced Industrial Science and Technology. The Office of Innovative Drug Discovery and Development began supporting prominent basic research results from universities to encourage the creation of innovative medicines. Then, upon establishment of AMED, the drug discovery support operations and employees were transferred to AMED, and the all-Japan drug discovery support system commenced.
Our undertaking is unique because, unlike research support in the past that focused mainly on research expenses, our triad of comprehensive support covers research and development strategy advice from a science coordinator with experience in drug discovery research in the pharmaceutical industry, technological support from the Drug Discovery Support Network, and the Contract Research Organization (CRO) expenses associated with optimization research and nonclinical tests. This setup provides seamless support starting from the results of basic research until the practical application as a pharmaceutical.
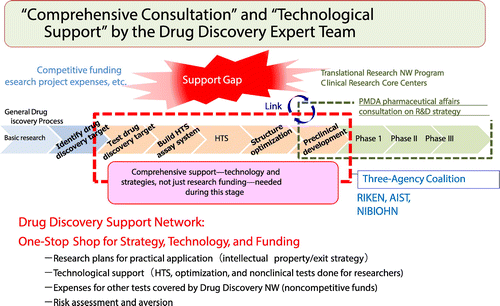
The structure of the Drug Discovery Support Network is shown in Fig. .Citation1) Overcoming the so-called “Valley of Death,” the gap between basic research and practical application, is not simple even for pharmaceutical companies. Financial support is often insufficient for academia to survive the “Valley of Death.” It is also necessary to have research strategies targeting practical application, the special technology and facilities essential for drug development, risk aversion, and the ability to advance a project by bringing people and organizations together. This is exactly what the Drug Discovery Support Network provides.
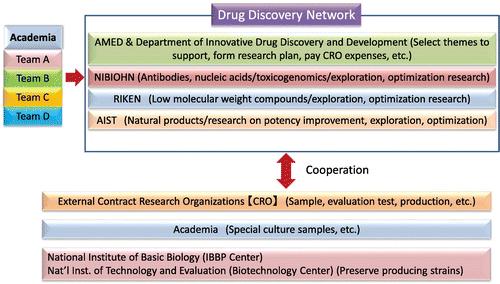
The Drug Discovery Support Network is a cross-ministry project involving the Ministry of Education, Culture, Sports, Science and Technology; the Ministry of Economy, Trade and Industry; and the Ministry of Health, Labor and Welfare. In addition, utilizing advanced technologies from the cooperative organizations RIKEN, the National Institute of Advanced Industrial Science and Technology, and the National Institute of Biomedical Innovation, Health and Nutrition, we work as a network to create all-Japanese pharmaceuticals based on the research results of academia. To fully utilize the available technology such as the high-throughput screening (HTS), in silico drug discovery, and optimization research capabilities at RIKEN; the HTS for natural product drug discovery and productivity enhancing capabilities for natural product compound-producing microorganisms at the National Institute of Advanced Industrial Science and Technology; and the screening capabilities for nucleic acids and antibodies at the National Institute of Biomedical Innovation, Health and Nutrition, experts at each institution are deeply involved in advancing the research as project members.
Drug discovery experts from the pharmaceutical industry are gathered as science coordinators in iD3. The science coordinators are responsible for collecting drug discovery seeds, providing their expert opinions on the seeds, planning and advising research and practical application strategies, and selecting technological support projects through the network. They also move projects forward in collaboration with academic researchers with proposed seeds. Support and advice concerning the establishment of intellectual property and company derivation are provided by the iD3. The ultimate goal of practical application is adoption by a company, but other options for the exit to practical application include investigator-initiated clinical trials and joint research with corporations.
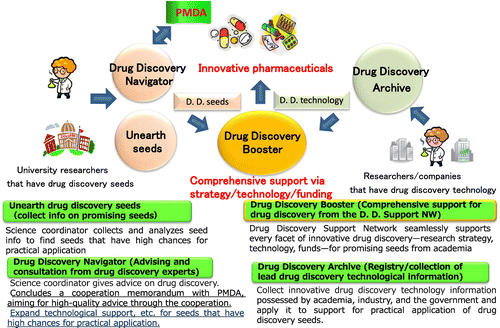
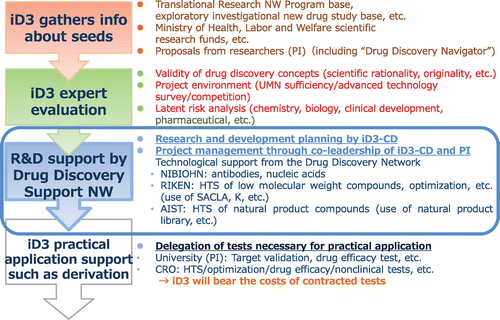
Fig. summarizes the initiatives undertaken by iD3. To unearth promising drug discovery seeds that will lead to innovative medicines, science coordinators gather information from various sources on research results produced by universities and public research institutions. Providing advice regarding drug discovery, “Drug Discovery Navigator” supports academia-generated drug discovery and aims to provide high-quality consultation by connecting and cooperating with the Pharmaceuticals and Medical Devices Agency (PMDA) when necessary. “Drug Discovery Archive” gathers various drug discovery support technologies retained by Japanese universities and corporations that are utilized to support the practical application of drug discovery seeds. “Drug Discovery Booster” makes full use of the drug discovery technology and facilities owned by the Drug Discovery Support Network constituent organizations and provides seamless support from HTS and nonclinical tests to the final step of company derivation. “Drug Discovery Booster” is the drug discovery comprehensive support initiative and the core of the Drug Discovery Support Network, comprising four sections: seed information collection, expert evaluation, research and development support, and practical application support (Fig. ). Seed information is gathered from various sources such as proposals from researchers, academic societies and literature, and the Ministry of Health, Labor and Welfare research funding applications.
The science coordinator performs an evaluation of all the gathered seed information to select seeds that should be supported by the “Drug Discovery Booster.” Once chosen, the seed (theme) begins to receive research and development support from the Drug Discovery Support Network. To advance the research, the Drug Discovery Support Network provides technological support and also delegates tests such as target suitability verifications and pharmacological drug efficacy tests for academia. We can also delegate nonclinical tests, including pharmacokinetic and safety studies, and the production of active pharmaceutical ingredient or drugs to external CROs. iD3 covers the contracted test expenses.
To date, the “Drug Discovery Booster” has organized and supported projects for 38 themes. The breakdown of the disease areas of the 38 themes is as follows: infectious disease 6, cancer 14, tissue regeneration 3, cardiovascular 5, neurodegenerative 1, psychiatric/neurological 1, gynecological 1, rare/specific disease 5, companion diagnostic 1, and other 1. Thus, we are promoting themes related to almost all areas of disease.
Efforts and issues of innovative drug development from natural products
According to a report by Newman et al.Citation2), over 50% of pharmaceuticals sold on the market (1355 drugs, 2012) originated from natural products or natural product derivatives. Natural products are a goldmine of substances with myriad chemical structures surpassing the capabilities of the human intellect. Of these, compounds derived from microbial metabolites have been utilized as an important resource for new medicines because microorganisms produce numerous compounds with biological activities that can be mass-produced through cell culture.
Currently, the system for utilizing natural product resources in academia-generated drug discovery seeds centers on iD3 (Fig. ). There are four research stages for drug discovery seeds supported by iD3: targeted practical application testing, screening, lead optimization, and preclinical development. Of the 38 themes mentioned earlier, natural product-associated fields include one infectious disease treatment drug, one in the lead optimization stage, and five in the screening stage. For the current antibiotic themes, the Drug Discovery Support Network provides comprehensive support. Not only do we support the research by delegating drug efficacy tests, safety studies, and pharmacokinetic studies to CROs, but we also manage strategies for and secure intellectual property rights and support studies to improve the productivity of production strains and technology, which are crucial for practical application.
From my experience, when aiming for practical application of drug discovery seeds from natural products, it is necessary to overcome the issues listed below. These problems create the image that natural product drugs have low acquisition efficiency and are recognized as the cause of the recent decline in natural product drug discovery research.
Problem 1: Acquiring and accumulating expertise on isolation and culture of strains is required, which takes effort.
Problem 2: A screening assay system for natural products must be constructed because samples for the assay contain contaminants.
Problem 3: Isolating the active substance and determining the structure requires time.
Problem 4: To obtain large quantities of the compound, time is required for improving the productivity of strains and establishing culture and purification methods.
Problem 5: It is necessary to secure natural product drug discovery researchers to pass down the technology.
Problem 6: There are high investment costs for jar fermenters and purification and analysis instruments.
Resolving the issues listed above is extremely difficult for academia alone. This is why having a system structured with the Drug Discovery Support Network at the core of an industry-academia-government coordination (Fig. ) is so vital to overcome those problems and advance natural product drug discovery research. In the future, it might be an effective solution to establish an organization like a specialized consortium for natural product drug discovery research comprising members from industry, academia, and the government.
Future developments
The future developments of natural product drug discovery research are the same in any era. Unique samples and stable assay methods serve as a platform for novel substance discoveries.
As noted above, there are still untapped drug discovery resources resting within the earth. Nevertheless, in January 2015, a newly developed culture method called “iChip” was announced in Nature by a European-American research team. They discovered the new compound “teixobactin,” which has a novel action, from the culture medium of a microorganism that is difficult to culture, Eleftheria terrae.Citation3) This example attests that novel substances can be discovered from natural products by utilizing new technology. Continuing to develop new culture methods like this and methods for heterologously expressing cloned gene clusters for secondary metabolite biosynthesis will be important for future acquisition of unique samples.
Furthermore, many compounds such as penicillin, avermectin, and tacrolimus, the pioneer of chemical biology, that were discovered by phenotypic assay were valued for their activity, strength, and uniqueness even though their target was unclear and established as medicines. HTS, with the concept of one gene one target, is approaching the limits of its drug discovery capabilities. Thus, by renewing our understanding of the complexity of in vivo reactions and through scientific advances in the noncoding DNA and RNA regions of the genome, the breadth of drug discovery targets has expanded, leading to reevaluation of phenotypic assays in recent years.Citation4) I believe that we can expect to obtain novel hits by combining phenotypic assays and whole-body assays that use individual organisms (Drosophila,Citation5) zebrafish,Citation6) silkworm,Citation7) etc.) which are not limited to drug discovery targets, with various ingenious, unique screening samples (e.g. extractive liquid-surface processing method.Citation8))
The movement of people due to globalization will be more active than ever in the years to come, and there is a high possibility that infectious NTDs and drug-resistant bacteria will become a familiar problem even in our country. Prof. Ōmura discovered an excellent medicine for onchocerciasis, but there is still great need for pharmaceuticals in this field of science. Depletion of novel compounds and cores is becoming a problem. Thus, it is thought that application of natural products in regions plagued by infectious diseases will be a key to future generation of compounds with novel backbones because the roots of most pharmaceuticals, including antibacterial agents, are in natural products. Drug-resistant bacteria are becoming a substantial social problem in the West as well as in Asia, and there is an urgent need for the development of treatment drugs. Against this background, the French company Sanofi and the German research institute the Fraunhofer Institute of Molecular Biology and Applied Ecology (IME) have been undertaking natural product exploratory research in the area of infectious diseases since 2014.
Furthermore, in the area of cutting-edge regenerative medicine, disease models using induced pluripotent stem cells for various illnesses that lack effective therapeutic methods continue to be constructed. It is believed that combining these with natural products will turn a new page in the story of natural product drug discovery.
In conclusion, natural products are expected to continue to play an important role as a source for drug discovery in the future. To meet the expectations of high-quality drug discovery seeds that are promising for practical application, we need to overcome each obstacle individually through cooperation between academia and industry. As a science coordinator, my goal is the production of all-Japanese medicines to show the world what we can achieve. Finally, I hope that those drugs contribute to the health of people worldwide and that many more researchers will follow in Prof. Ōmura’s footsteps.
Disclosure statement
No potential conflict of interest was reported by the author.
Notes
† This work is a translation of an original work in Japanese in the Japan Society for Bioscience, Biotechnology, and Agrochemistry http://doi.10.1271/kagakutoseibutsu.54.43
References
- Takashi T. Kokusai Iryohin Joho 3. 2013; [in Japanese].
- Newman DJ, Cragg GM. J Nat Prod. 2012;75:311.10.1021/np200906s
- Ling LL, Schneider T, Peoples AJ, et al. Nature. 2015;517:455.
- Swinney DC, Anthony J. Nat. Rev Drug Discov. 2011;10:507.10.1038/nrd3480
- Insect Biomedical Research Center. Available from: http://kit-ibrc.com/
- Medical Zebrafish Research Consortium. Available from: http://pgx.medic.mie-u.ac.jp/mzrc/
- Hamamoto H, Sekimizu K. Seikagaku. 2014;86:578.
- Oda S, Kameda A, Okanan M, et al. J Antibiot (Tokyo). 2015;68:691.10.1038/ja.2015.59