Abstract
A novel indole derivative (1) and three known compounds (2–4) were isolated from the fruiting bodies of Tricholoma flavovirens. Their structures were determined or identified by the interpretation of spectroscopic data. Compounds 1 and 2 promoted root growth of lettuce and inhibited hypocotyl growth at 1 μmol/paper. Compound 3 inhibited hypocotyl and root growth at 100 nmol/paper.
We have been continuing to search for bioactive compounds from mushrooms using various bioassays. In our previous works, we have isolated several plant growth regulators from several kinds of fruiting bodies or culture broth of higher fungi.Citation1–3)
We also reported the isolation and structural determination of a novel compound and a known one from the mushroom Tricholoma flavovirens.Citation4) During further search, we succeeded in isolating plant growth regulating compounds from this mushroom.
Here we describe the isolation and structural determination of a novel indole derivative and three known compounds, and their activity.
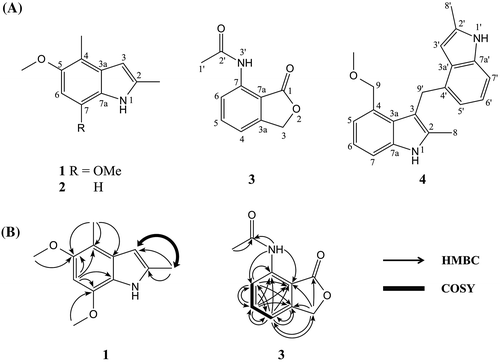
Fresh fruiting bodies of T. flavovirens were extracted with EtOH and then with acetone. After the solutions were combined and concentrated, they were partitioned between n-hexane and H2O, EtOAc and H2O, and then n-BuOH and H2O. The n-hexane- and EtOAc-soluble parts were fractionated by repeated chromatography. As a consequence, four compounds (1–4) were purified (Fig. (A)).
Compound 1 was isolated as a white amorphous, mp 178–180 °C (decomp.). Its molecular formula was determined as C12H15NO2 by HRESIMS at m/z 206.1165 [M + H]+ (calcd. for C12H16NO2 206.1181), indicating the presence of six degrees of unsaturation in the molecule. The structure of 1 was elucidated by interpretation of NMR spectra including DEPT, COSY, HMQC, and HMBC (Fig. (B)). The DEPT experiment indicated the presence of four methyls, two methines and six quaternary carbons. The structure of 2,4-dimethylindole skeleton was elucidated by the HMBC correlations (H-2-Me/C-2, C-3; H-4-Me/C-3a, C-4, C-5, H-6/C-4, C-5, C-7, C-7a) and the COSY correlations (H-2-Me/H-3). The HMBC correlations (H-5-OMe/C-5; H-7-OMe/C-7) indicated the position of 5-OMe and 7-OMe on the 2,4-dimethylindole skeleton. The complete assignment of protons and carbons of NMR was accomplished as shown in Table . As a result, the structure of 1 was determined to be 5,7-dimethoxy-2,4-dimethylindole.
Table 1. 1H and 13C NMR Data for 1 and 3 (in CDCl3).
Compound 2 was isolated as a white amorphous. It was identified as 5-methoxy-2,4-dimethylindole. This compound has been reported as a degradation product when the bitter principle of Tricholoma lascivum, lascivol, was treated with strong acid, and has been isolated from the same genus mushroom Tricholoma sciodes.Citation5,6)
Compound 3 was isolated as a white crystal. Its molecular formula was determined as C10H9NO3 by HRESIMS at m/z 190.0510 [M − H]− (calcd. for C10H8NO3 190.0504), indicating the presence of seven degrees of unsaturation in the molecule. The structure of 3 was elucidated by interpretation of NMR spectra including DEPT, COSY, HMQC, and HMBC (Fig. (B)). The DEPT experiment indicated the presence of one methyl, four methines, and five quaternary carbons. The structure of phthalide skeleton was elucidated by the HMBC correlations (H-3/C-1, C-3a, C-4, C-7a; H-4/C-3, C-3a, C-5, C-6, C-7, C-7a; H-5/C-3a, C-4, C-6, C-7, C-7a; H-6/C-3a, C-4, C-5, C-7, C-7a) and the COSY correlations (H-4/H-5, H-5/H-6). The HMBC correlations (H-1′/C-2′; H-3′/C-2′) indicated the presence of acetamido group. The connection between acetamido group and phthalide was confirmed by the HMBC correlations (H-3′/C-6, C-7a). The complete assignment of protons and carbons of NMR was accomplished as shown in Table . As a result, 3 was identified to be 7-acetamidophthalide. This compound has been synthesized, but this is the first report as a natural compound.Citation7)
Compound 4 was isolated as a white amorphous. It was identified as 4-methoxymethyl- 3-[(2-methyl-4-indolyl)methyl]-2-methylindole. It has also been isolated from T. sciodes together with 2.Citation6)
Biological activities of compounds 2–4 have not been reported yet.
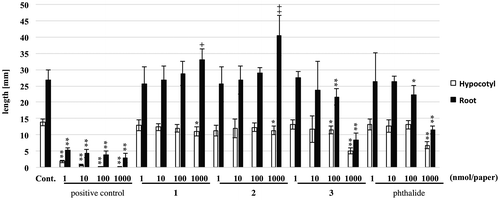
Compounds 1–3 were evaluated in the plant growth regulatory assay using lettuce (Fig. ). 2,4-Dichlorophenoxyacetic acid was used as positive control, which inhibited the hypocotyl and root growth of lettuce dose dependently. In order to know structure–activity relationship, 3 was compared with phthalide. As a result, 1 and 2 promoted the root growth and inhibited hypocotyl growth at 1 μmol/paper. 3 and phthalide inhibited the root growth dose dependently. In addition, phthalide inhibited the hypocotyl growth at 1 μmol/paper, while 3 showed inhibition activity at 100 nmol/paper.
Fig. 2. Growth-regulating activity against lettuce of compounds 1–3.
1H NMR spectra (one-and two-dimensional) were recorded on a Jeol lambda-500 spectrometer (Jeol Ltd., Tokyo, Japan) at 500 MHz, while 13C NMR spectra were recorded by the same instrument at 125 MHz. HRESIMS data were measured by a JMS-T100LC mass spectrometer (Jeol Ltd., Tokyo, Japan). HPLC separation was performed with a Jasco Gulliver system (Jasco Co., Tokyo, Japan) using a reverse-phase HPLC column (Cosmosil πNAP Waters, 10 × 250 mm, Nacalai tesque, Kyoto, Japan) and two normal phase HPLC columns (YMC-pack Diol-60-NP, 20 × 250 mm, YMC Co., Ltd., Kyoto, Japan; Senshu Pak AQ, 20 × 250 mm, Senshu Scientific Co., Ltd., Tokyo, Japan). Silica cartridges and C18 cartridges (Nihon Waters K.K., Tokyo, Japan) were used in the pro-processing of the samples. Silica gel plate (TLC Silica gel 60 F254, Merck KGaA, Darmstadt, Germany) and silica gel 60 N (Kanto Chemical Co., Inc., Tokyo, Japan) were used for analytical TLC and for flash column chromatography, respectively.
Fresh fruiting bodies of T. flavovirens were collected at Narusawa village, Yamanashi Prefecture in Japan. Lettuce seeds (Lactuca sativa L. cv. Great Lakes 366; Takii Co., Ltd., Tokyo, Japan) were used in this study.
The fresh fruiting bodies of T. flavovirens (20.6 kg) were extracted with EtOH (42 L, 3 times) and then with acetone (20 L, 3 times). After the solutions were combined and concentrated under reduced pressure, the concentrate was partitioned between n-hexane and H2O, EtOAc and H2O, and then n-BuOH and H2O. The n-hexane-soluble part (39.8 g) was fractionated by silica gel flash column chromatography (CH2Cl2; 90/10, 80/20 CH2Cl2/acetone; 90/10, 80/20 CH2Cl2/MeOH; MeOH; 95/5 MeOH/H2O; 2.0 L each) to obtain 20 fractions (fractions 1–20). Fraction 7 (6.78 g) was further separated by silica gel flash column chromatography (CH2Cl2; 95/5, 90/10, 80/20 CH2Cl2/acetone; MeOH; 2 L each) to give 14 fractions (fractions 7-1 to 7-14). Fraction 7-4 (23.3 mg) was further separated by normal-phase HPLC (YMC-pack Diol-60-NP, UV 245 nm, 5 mL/min, 30/70 hexane/CHCl3) to afford 1 (1.5 mg). Fraction 7-3 (28.5 mg) was separated by reverse-phase HPLC (Cosmosil πNAP Waters, UV 254 nm, 2 mL/min, 80/20 MeOH/H2O) to afford 2 (1.5 mg).
The EtOAc-soluble part (16.4 g) was fractionated by silica gel flash column chromatography (CH2Cl2; 95/5, 90/10, 80/20, 70/30, 50/50 CH2Cl2/EtOAc; MeOH; 2L each) to obtain 17 fractions (fractions 1–17). Fraction 8 (426 mg) was fractionated by C18 cartridges to give two fractions (fractions 8-1 and 8-2). Fraction 8-1 (214 mg) was separated by silica gel flash column chromatography (CH2Cl2; 95/5, 90/10, 80/20 CH2Cl2/acetone; MeOH; 500 mL each) to give 8 fractions (fractions 8-1-1 to 8-1-8). Fraction 8-1-5 was further fractionated by preparative TLC to give 10 fractions (fractions 8-1-5-1 to 8-1-5-10). Fraction 8-1-5-1-5 (10.9 mg) was separated by reverse-phase HPLC (Cosmosil πNAP Waters, UV 255 nm, 2 mL/min, 75/25 MeOH/H2O) to afford 3 (7.7 mg). Fraction 10 (740 mg) was fractionated by silica gel flash column chromatography (CH2Cl2; 90/10 CH2Cl2/acetone; MeOH; 500 mL each) to give 11 fractions (fractions 10-1 to 10-11). Fractions 10-4 (5.5 mg), 10-5 (7.2 mg) and 10-6 (20.5 mg) were separated by normal-phase HPLC (Senshu Pak AQ, UV 270 nm, 5 mL/min, 70/30 hexane/CHCl3), respectively, to afford 4 (0.8 mg) in total.
Plant growth regulating activity against lettuce was examined as follows. Lettuce seeds were put on filter paper (Advantec No. 2, ϕ 55 mm; Toyo Roshi Kaisha, Ltd., Japan), soaked in distilled water in a Petri dish (ϕ 60 × 20 mm), and incubated in a growth chamber under dark at 25 °C for 1 day. Each sample was dissolved in 1 mL of methanol (1, 10, 102, and 103 nmol/mL) and then poured on filter paper (ϕ 55 mm) in a Petri dish (ϕ 60 × 20 mm). After the solvent was air-dried, 1 mL of distilled water was poured on the sample-loaded paper or intact filter paper (control). The pre-incubated lettuces (n = 9 in each petri dish) were transferred onto the filter paper and incubated in a growth chamber under dark at 25 °C for 3 days. The lengths of the hypocotyl and the root were measured using a ruler.
Author contributions
Hirokazu Kawagishi designed the experiments. Weitao Qiu, Hajime Kobori, and Jing Wu performed the experiments. Jea-Hoon Choi, Hirofumi Hirai, and Hirokazu Kawagishi contributed to discussions. Weitao Qiu and Hirokazu Kawagishi wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by authors.
References
- Fushimi K, Anzai K, Tokuyama S, et al. Agrocybynes A–E from the culture broth of Agrocybe praecox. Tetrahedron. 2012;68:1262–1265.10.1016/j.tet.2011.11.049
- Wu J, Tokunaga T, Kondo M, et al. Erinaceolactones A to C, from the culture broth of Hericium erinaceus. J Nat Prod. 2015;78:155–158.10.1021/np500623s
- Kobori H, Sekiya A, Suzuki T, et al. Bioactive sesquiterpene aryl esters from the culture broth of Armillaria sp. J Nat Prod. 2015;78:163–167.10.1021/np500322t
- Qiu W, Kobori H, Suzuki T, et al. A new compound from the mushroom Tricholoma flavovirens. Biosci Biotechnol Biochem. 2014;78:755–757.10.1080/09168451.2014.905174
- Eizenhöfer T, Fugmann B, Sheldrick WS, et al. Lascivol, the bitter component of Tricholoma lascivum (Agaricales). Liebigs Ann Chem. 1990;11:1115–1118.10.1002/(ISSN)1099-0690
- Pang Z, Sterner O. Novel indole derivatives from the fruit bodies of Tricholoma sciodes. Acta Chem Scand. 1996;50:303–304.10.3891/acta.chem.scand.50-0303
- Vene J, Tirouflet J. Synthesis of 4-amino- and 7-aminophthalide and some of their derivatives. Compt Rend. 1950;231:911–912.