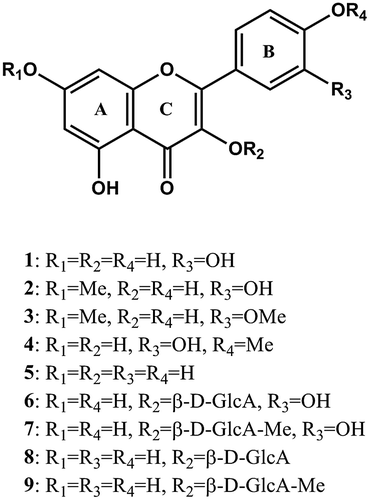
Abstract
O-Methylated and glucuronosylated flavonoids were isolated from Tamarix gallica as α-glucosidase inhibitors. Structure–activity relationship of these flavonoids suggests that catechol moiety and glucuronic acid at C-3 are factors in the increase in α-glucosidase inhibitory activity. Furthermore, rhamnetin, tamarixetin, rhamnazin, KGlcA, KGlcA-Me, QGlcA, and QGlcA-Me exhibit synergistic potential when applied with a very low concentration of acarbose to α-glucosidase from rat intestine.
Diabetes mellitus (DM) is a leading and serious health concern worldwide. Type 2 diabetes (T2D) accounts for more than 90% of all cases of diabetes globally.Citation1) Despite numerous pharmaceutical strategies for the treatment of diabetes, its incidence continues to increase. The management of postprandial hyperglycemia is considered to be a major therapeutic strategy.Citation2) This can be achieved by delaying the release of glucose through the inhibition of the carbohydrate hydrolyzing enzyme α-glucosidase (EC 3.2.1.20) in the digestive tract.Citation3,4) Commercial α-glucosidase inhibitors, such as acarbose, have associated side effects, including liver disorders and acute hepatitis.Citation5–7) Therefore, identifying other α-glucosidase inhibitors is important. Supplementation with antioxidants can be beneficial for diabetic patients, not only to maintain antioxidant levels in the body but also to treat long-term complications that can arise.Citation8) The powerful antioxidant activity of medicinal halophyte Tamarix gallica L. has been reported as exhibiting a remarkable spectrum of pharmacological activities. Although, traditionally used for the treatment of various liver disorders and marketed as a herbal medicine in many countries,Citation9–11) there have been no reports on the use of this plant for the treatment or prevention of T2D. The objectives of this study are to isolate the α-glucosidase inhibitory substances from this halophyte, to investigate their kinetic activity, the flavonoid structure required to inhibit these digestive enzymes and, finally, to study their synergistic effects.
Dried and crushed aerial parts (200 g) of T. gallica were extracted with 70% ethanol. After filtration, solvent of the extract was evaporated, and partitioned the residue into CHCl3 (200 mL × 2), EtOAc (200 mL × 3), and H2O (200 mL). The CHCl3 layer (TGC, 1.1 g) was chromatographed on a silica gel column (ϕ 3.0 × 35 cm, Nacalai Tesque, Inc., Japan) with acetone–hexane (0:100 → 100:0), which yielded eight fractions, TGC-1-8. TGC-5 (299.4 mg) eluted with acetone–hexane (40:60) was purified by octadecyl-silane (ODS) HPLC [TSKgel ODS-80Ts (ϕ 4.6 × 250 mm, Tosoh Corporation, Japan), flow rate 1.0 mL/min; MeCN/H2O–0.1%TFA (5:95 (5 min) → 30:70 (5 min) → 40:60 (40 min) → 40:60 (10 min) → 55:54 (2 min) → 100:0 (2 min))] to yield quercetin (1, 1.2 mg, tR 43 min), rhamnetin (2, 2.9 mg, tR 46 min), and rhamnazin (3, 1.6 mg, tR 72 min). Tamarixetin (4, 2.0 mg, tR 37 min) was purified from TGC-6 (100.1 mg) using ODS HPLC [TSKgel ODS-80Ts (ϕ 4.6 × 250 mm), flow rate 1.0 mL/min; MeCN/H2O–0.1%TFA (5:95 (5 min) → 30:70 (25 min) → 40:60 (10 min) → 40:60 (10 min) → 55:54 (2 min) → 100:0 (2 min))]. The EtOAc-soluble portion (TGE, 987 mg) was eluted on an ODS column (Cosmosil 75 C18-PREP, ϕ 3.0 × 35 cm, Nacalai Tesque, Inc., Japan) with MeOH/H2O (10:9 → 100:0), yielding six fractions, TGE-1-6. TGE-6 (228.3 mg) eluted with MeOH/H2O (100:0) was fractionated by ODS HPLC [TSKgel ODS-80Ts (ϕ 4.6 × 250 mm), flow rate 1.0 mL/min; MeOH/H2O–0.1%TFA (5:95 (5 min) → 55:54 (55 min) → 85:15 (2 min) → 100:0 (2 min))] to yield eight sub-fractions. TGE-6-8 was identified as kaempferol (5, 3.3 mg, tR 37.5 min). TGE-6-3 (24.7 mg, tR 28.1 min) was purified by ODS HPLC [TSKgel ODS-80Ts (ϕ 4.6 × 250 mm), flow rate 1.0 mL/min; MeCN/H2O (5:95 (5 min) → 25:75 (41 min))] to yield quercetin 3-O-β-D-glucuronide (QGlcA) (6, 6.3 mg, tR 9.3 min), and quercetin 3-O-β-D-glucuronide methyl ester (QGlcA-Me) (7, 9.0 mg, tR 17.6 min). And, TGE-6-5 (20 mg, tR 32.3 min) was also purified by ODS HPLC [TSKgel ODS-80Ts (ϕ 4.6 × 250 mm), flow rate 1.0 mL/min; MeCN/H2O (5:95 (1 min) → 30:70 (45 min))] to yield kaempferol 3-O-β-D-glucuronide (KGlcA) (8, 2.0 mg, tR 11.5 min), and kaempferol 3-O-β-D-glucuronide methyl ester (KGlcA-Me) (9, 5.0 mg, tR 12.3 min). The structure of these substances was confirmed by NMR (Avance 500, Bruker, Germany) spectral analyses (supp. data) and compared with the literature.Citation12–14)
The α-glucosidase inhibition potential of the purified flavonoids 1–9 was measured at concentrations of 1, 10, and 100 μM, using disaccharide assay as follows: mammalian α-glucosidase was prepared by homogenizing 100 mg of rat intestinal acetone powder in 3 mL of 0.9% NaCl solution (5000 g × 30 min). The reaction mixture comprising from 50 μL of 0.1 M phosphate buffer (pH 6.8) and 10 μL of the sample at various concentrations was mixed with 35 μL of maltose solution (5 mM) for maltase inhibition test, and 20 μL of sucrose (56 mM) for sucrase inhibition test. After pre-incubation for 5 min at 37 °C, the enzyme solution was added, 20 μL in case of sucrase inhibition assay and 5 μL for maltase inhibition assay, and incubated with the mixture for 15 min at 37 °C. To stop/suspend the reaction, 2 M Tris–HCl buffer (75 μL) was added to the reaction mixture. The concentrations of glucose released from the reaction mixtures were determined using glucose oxidase method. The enzyme activity was quantified by measuring the absorbance at 490 nm in a microtiter plate reader (Bio-TEK, USA). Acarbose was used as a positive control. All experiments were performed in triplicate and the percentages of enzyme inhibition were calculated using the following formula: % inhibition = [(AC – AS)/AC] × 100,Citation15) where AC is the absorbance of the control and AS is the absorbance of the tested sample. α-Glucosidase inhibition potential of the purified flavonoids 1–9 are displayed in Table . Rhamnetin (2), rhamnazin (3), QGlcA (6), QGlcA-Me (7), KGlcA (8), and KGlcA-Me (9) are reported for the first time as α-glucosidase inhibitors.
Table 1. α-Glucosidase inhibition activity, inhibition mode and specificity for sugar-based substrates using mammalian enzyme.
Each of the purified flavonoids 1–9 showed a dose-dependent inhibition activity (Table ). Among all the tested flavonoids 1–9, compounds 6–9 show relatively strong inhibitory activity against maltase at 100 μM. The results suggest that the substitution of the hydroxyl group at the C-3 position with a glucuronic acid and its methyl ester significantly enhanced the inhibition activity of 1 and 5 toward α-glucosidase. There have been no report of the relationship between α-glucosidase inhibitory activity and glucuronide moiety thus far (see Fig. ).
The mode of inhibition was determined using the enzyme kinetic assay with increasing concentrations of p-nitrophenol α-D-glucopyranoside (p-NPG) (0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 16.0 mM) as a substrate in the presence or absence of inhibitors. Lineweaver–Burk (LB) plot analysis was conducted and the data were calculated following Michaelis–Menten kinetics. The double-reciprocal plot displayed mixed inhibition for 1, 2, 3, 4, and 5; and non-competitive inhibition for 6, 7, 8, and 9 (Table ).
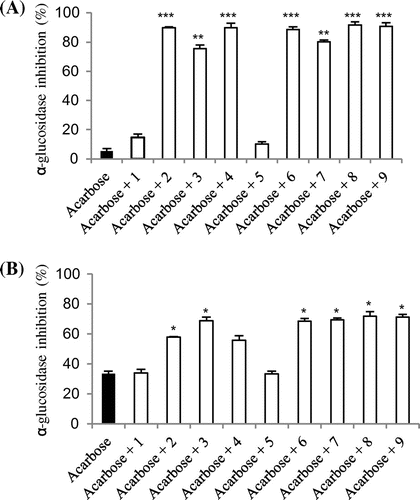
A recent report has shown that the lowest dose of acarbose with clinical effects is 150 mg/day, with doses >300 mg/day exceeding the saturated binding of α-glucosidase.Citation16,17) It has also been reported that the most common adverse effect of acarbose is gastrointestinal disturbance, which is dose-dependent.Citation18) Therefore, it was of interest to determine whether O-methylated flavonoids 2–4 or glucuronosylated flavonoids 6–9 and acarbose might interact synergistically in intestinal α-glucosidase inhibition. Acarbose was combined with and without the purified flavonoids 1–9 at low concentration (1 μM). For this reaction, the same procedure as in the disaccharide assay was followed and results were expressed as a percentage inhibition of the corresponding control values. The results of this experiment are shown in Fig. and reveal that when O-methylated flavonoids 2–4 or glucuronosylated flavonoids 6–9 were added to the assay system with acarbose, the percentage of intestinal maltase and sucrase inhibition, respectively, increased as compared with using acarbose alone. Thus, it is possible that mixing flavonoids with acarbose may provide a significant clinical benefit in delaying postprandial hyperglycemia. And as a consequence, it would be possible to reduce the dosage of acarbose. However, further clinical investigation is necessary.
Author contributions
A. Ben Hmidene and H. Shigemori designed the research. A. Ben Hmidene performed the experiments. A. Ben Hmidene wrote the draft of manuscript and H. Shigemori supervised manuscript preparation. All authors reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was partially supported by the JST-JICA Science and Technology Research Partnership for Sustainable Development (SATREPS) and grants-in-aid for the Ministry of Education, Science, Sports, and Culture of Japan.
Supplemental material
The underlying research materials for this article can be accessed at http://dx.doi.org/10.1080/09168451.2016.1254538.
TBBB_1254538_Supplementary_Material.docx
Download MS Word (20.4 KB)References
- Kimmel B, Inzucchi S. Oral agents for type 2 diabetes: an update. Clin Diabetes. 2005;23:64–76.10.2337/diaclin.23.2.64
- Woerle HJ, Pimenta WP, Meyer C, et al. Diagnostic and therapeutic implications of relationships between fasting, 2-h postchallenge plasma glucose and hemoglobin A1c values. Arch Intern Med. 2004;164:1627–1632.10.1001/archinte.164.15.1627
- Casirola DM, Ferraris RP. α-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism. 2006;55:832–841.10.1016/j.metabol.2006.02.011
- Zhang L, Hogan S, Li JR, et al. Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mice. Food Chem. 2011;126:466–471.10.1016/j.foodchem.2010.11.016
- Madar Z. The effect of acarbose and miglitol (BAY-M-1099) on postprandial glucose levels following ingestion of various sources of starch by non-diabetic and streptozotocin-induced diabetic rats. J Nutr. 1989;119:2023–2029.
- Murai A, Iwamura K, Takada M, et al. Control of postprandial hyperglycaemia by galactosyl maltobionolactone and its novel anti-amylase effect in mice. Life Sci. 2002;71:1405–1415.10.1016/S0024-3205(02)01844-1
- Fujisawa T, Ikegami H, Inoue K, et al. Effect of two α-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism. 2005;54:387–390.10.1016/j.metabol.2004.10.004
- Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;56:2806–2821.
- Tabassum N, Chatturvedi S, Agrawal SS. Effect of Tamarix gallica leaves on experimental liver cell injury. JK-Pract. 2016;13:43–44.
- Ksouri R, Falleh H, Megdiche W, et al. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem Toxicol. 2009;47:2083–2091.10.1016/j.fct.2009.05.040
- Mayuresh R, Andrzej P, Patrycja LK, et al. Herbal medicine for treatment and prevention of liver diseases. J Pre-Clin Clin Res. 2014;8:55–60.
- Saewan N, Koysomboom S, Chantrapromma K. Anti-tyrosinase and anti-cancer activities of flavonoids from Blumea balsamifera DC. J Med Plants Res. 2011;18:1018–1025.
- Paya M, Manez S, Villar A. Flavonoid constituents of Rhamnus lycioides L. Z. Naturforsch. 1986;41c: 976–978.
- Nawwar MAM, Souleman AMA, Buddrus J, et al. Flavonoids of the flowers of Tamarix nilotica. Phytochemistry. 1984;23:2347–2349.10.1016/S0031-9422(00)80549-X
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108.
- Rodier M, Richard JL, Monnier L, et al. Effect of long term acarbose (Bay g 5421) therapy on metabolic control of non insulin dependent (type II) diabetes mellitus. Diabetes Metab. 1988;14:12–14.
- Santeusanio F, Ventura MM, Contadinbi S. Efficacy and safety of two different dosages of acarbose in non-insulin dependent diabetic patients treated by diet alone. Diabetes Nutr Metab. 1993;6:147–154.
- Hanefeld M, Fischer S, Schulze J, et al. Therapeutic potentials of acarbose as first-line drug in NIDDM insufficiently treated with diet alone. Diabetes Care. 1991;14:732–737.10.2337/diacare.14.8.732