Abstract
Oryzanol contained in rice bran is a complex mixture of steryl ferulates (SFs) with many identified health benefits. Recently, SF has been shown to exist in other cereals such as wheat, rye, and corn. In this study, SFs in several wheats produced in Japan were analyzed. For instance, SF content of whole wheat grain, Yumekaori (Japan) was 15.2 ± 1.4 mg-oryzanol-equivalent/100 g grain, while that of the imported one, 1CW (Canada) was 11.4 ± 1.3 mg-oryzanol-equivalent/100 g grain. The main SF components in the examined wheats were campesteryl ferulate, campestanyl ferulate, and sitostanyl ferulate. SF distribution in whole wheat grain was investigated using 14 fractions produced by a conventional test milling machine. SF was intensively accumulated in the four bran fractions (24 − 95 mg-oryzanol-equivalent/100 g bran fraction). These results suggest that the wheat bran would be an important source of SF.
Cereals are important dietary sources of natural phytosterols and their conjugates. In several cereals, some of phytosterols and triterpene alcohols are esterified with ferulic acid. Thus, conjugate has been known as a steryl ferulate (SF).Citation1) In addition to the bioactive aspect of a phytosterol conjugate, SF possesses a free radical scavenging activity by virtue of the phenolic hydroxyl group of ferulic acid.Citation2) Therefore, SF is also an effective antioxidant compound and its therapeutic property has been evaluated in a series of investigations.Citation3,4)
The most famous SF is oryzanol (γ-oryzanol), which was first found from rice bran oil in 1954 by Kaneko and TsuchitaCitation5). Recently, 24 components of SF in the oryzanol fraction from crude rice bran have been successfully identified,Citation6) and 24-methylene cycloartanyl ferulate, cycloartenyl ferulate, campesteryl ferulate, and β-sitosteryl ferulate are principal components of oryzanol.Citation6–8) But the exact SF composition of oryzanol depends on the rice cultivar.Citation9) Although the functions of oryzanol in brown rice grain are not fully understood, the previous studies suggest that oryzanol is involved in the physical and chemical defenses against various micro-organisms.Citation2,10) While there have been many studies on the physiological properties of oryzanol, including the superoxide dismutase-like antioxidant activities and antihypercholesterolemic effects found in animal models.Citation11,12) Oryzanol has been admitted as a food additive since 1995 in Japan. And several studies show that intake of oryzanol is associated with the reductions in the risks of metabolic diseases, including protections against cardiovascular disease, type 2 diabetes, cancer, and obesity, and resulting improvements of human health.Citation4,13) Because of these therapeutic properties, oryzanol has been used as a potential additive, not only in various food products, but also in pharmaceuticals and cosmetics.Citation14)
In addition to oryzanol of rice bran, SFs have been found in other cereals such as wheat, corn, rye, and triticale.Citation15) In the large EU project HEALTHGRAIN, a number of bioactive components contained in cereals were analyzed with the aims to enhance industrial usage of whole grain and to derive these bioactive components from appealing agricultural products. In this project, localizations and compositions of SF in wheat and rye have been investigated,Citation12,14,16) because these cereals are predominantly consumed in European countries. For example, SF accounted for 6–10% of the total sterols in wheat and 4–5% in rye.Citation14) Although phytosterols are known to accumulate in the bran and germ fractions in whole wheat grain, SF contributed up to 17% of total phytosterols in the bran.Citation17) In wheat, sitostanol, campesterol, and campestanol are abundant phytosterols, and these phytosterols occur predominantly in the components of SF.Citation17) The level of SF content in whole wheat grain is estimated in the range from 6.6 to 12.6 mg/100 g grain.Citation12,16) Furthermore, these previous studies reveal that the SF contents in the cereals are varied depending on genetic and environmental factors.Citation14,16)
Whole wheat grain is rich not only in SF, but also in dietary fiber, minerals, phytochemicals, and their derivatives. However, following wheat milling, the outer parts of the wheat grains are removed from the flour products. Only refined white endosperm-based flour is generally used as the materials of various food products and the bran fraction is regarded as a by-product. For example, during a milling process with an 80% extraction rate, a large part of the beneficial compounds located in the bran fraction is discarded or ends up as animal feed. Previous studies have shown that various milling by-products will become sources of these beneficial compounds.Citation12,16) Therefore, knowing the exact distribution of SF among the milling fractions of wheats used in Japan is important for its effective usage.
Since rice is the staple food in Japan, rice bran is mass produced compared to other cereals. Most SF produced in Japan is extracted from rice bran, driving an economic boost in rice farming and processing. In addition, SF content in rice bran is much than those of other cereals.Citation16) For these reasons, SF in wheat has received little attention in Japan, and it has not been widely used in the industrial field. Alternatively, physiological studies using SF components originated from wheats have not been proceeded.
In this study, the distribution and content of SF in the wheats produced in Japan and those in the wheats imported and used in Japanese food products were analyzed. The SF content of commercially available flours, in which the outer parts of the wheat are removed, was also investigated. Furthermore, one of the Japanese wheat, Yumekaori and an imported variety, 1CW were milled using a conventional test-mill machine and the milling fractions which were rich in SF were identified in order to exploit their industrial usage as an SF source with beneficial health effects.
Materials and methods
Materials
Whole wheat grains (Triticum aestivum L.) used in this study were provided by Chiba Flour Milling Co. Ltd. (Chiba, Japan), Yokoyama Flour Milling Co. Ltd. (Sapporo, Japan), and Ibaraki Agriculture Research Center (Mito, Japan). Japanese wheat varieties were harvested in 2015 in Hokkaido (Haruyokoi, Kitahonami, Yumechikara, Kitanokaori, Harukirari, Haruyutaka), Gunma (Satonosora), Saitama (Hanamanten), and Ibaraki (Yumekaori). In case of Yumekaori, wheat seeds were harvested in Chikusei-shi, Ibaraki in June 2015. After dehulled and dried in the field, whole wheat grains were stored in agricultural cooperative warehouse under no controls of temperature and humidity for several weeks. Then, they were transferred to the milling company and stored for six months in the warehouse, in which temperature and humidity were not controlled. Brown rice seeds (Oryza sativa var. japonica Koshihikari) were harvested in September 2015 in Ibaraki, Japan and stored in a grain storage. The additional imported wheat varieties, Western Red Spring (1CW), Western White (WW), Hard Red Winter (SH), Dark Northern Spring (DNS), Standard White (ASW), and Prime Hard (PH) were harvested and dehulled in 2015 in Canada (1CW), USA (WW, SH, DNS), and Australia (ASW, PH), and imported to Japan under Japanese governmental control. As far 1CW, the seeds were harvested in September 2015 in Canada. After dehulled and dried, they were transferred to Japan by shipping for several weeks. The imported whole wheat grains were stored in the silo with no controls of temperature and humidity. After 2 or 3 months’ storage, they were transferred to the milling company. The commercially available flours, Kameriya and Savory (Nissin Seifun Group Inc. Tokyo, Japan) were purchased at local stores in Ibaraki, Japan.
Samples of milling fractions of wheats (1CW and Yumekaori) were prepared using a small-scale automatic milling unit (test-mill), the Miaγ Λaboratory Mill “Multomat” (Miag, Braunschweig, Germany). Samples of approximately 4000 g were taken from streams originating from the outer parts of the kernels of each cultivar and were separated into 14 fractions. After fractionation, samples were stored at −20 °C until analysis.
Reagents
The oryzanol standard was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). For the HPLC solvents and SF extraction from grains, analytical grade n-hexane, acetonitrile, 2-propanol, tert-buthyl methyl ether (t-BME), and ethyl acetate were obtained from Wako Pure Chemical Industries, Ltd. and Kanto Chemical Co., Inc. (Tokyo, Japan). All other chemical reagents were obtained from Wako Pure Chemical Industries, Ltd. and Kanto Chemical Co., Inc.
Extractions of SFs from whole wheat grain and brown rice
Extractions of SFs from whole wheat grain and brown rice were performed according to Esche et al.Citation18) with modifications to the sample size and the number of extractions. For each grain sample, about five-gram samples of whole kernels were milled to a powder using a domestic miller (Iwatani, IFM-800DG, Osaka, Japan) and a mortar. Samples that were powdered in advance were used directly for SF extraction. Two-gram powdered samples were precisely weighted in a glass vessel. After the addition of a magnetic stir bar, SF was extracted with 10 mL of n-hexane/2-propanol mixture (9:1, v/v) with stirring for 1 h at 4 °C. After filtration, the extraction vessel and the filter was washed twice with n-hexane/2-propanol solution. The combined filtrates were evaporated to dryness by rotary evaporation. The residue was dried to a constant weight. Then, 50 mg of the obtained residues were dissolved in 5 mL of n-hexane and used for solid phase extraction (SPE).
Solid phase extraction
The lipid extracts were separated by SPE (Strata, NH2, 55 μm, 70 Å, 1 g/6 mL, Phenomenex, Torrance, CA, USA), as previously described.Citation18) One milliliter of the extraction solution was loaded onto the SPE cartridge that had been conditioned with 2 × 5 mL of n-hexane and 2 × 5 mL of n-hexane/diethyl ether (98:2, v/v). Interfering triacylglycerols were removed with 4 × 5 mL of n-hexane/ethyl acetate (96:4, v/v). After eluting free sterols and stanols with 2 × 5 mL of n-hexane/ethyl acetate (5:95, v/v), SFs were eluted with 2 × 5 mL n-hexane/ethyl acetate (5:95, v/v), followed by 2 × 5 mL t-BME. The SF fraction solvent was evaporated by rotary evaporation. The residues were dissolved in 500 μL of ethanol for HPLC analysis. An addition recovery test for SPE separation was not done, because there were no suitable reagents as internal standards. One milliliter of commercially available oryzanol standard solution (50 μg/mL) was applied on the same SPE system and its recovery was 96.4 ± 2.6%.
HPLC analysis
SF analyses were conducted by reverse phase high-performance liquid chromatography using a C18-column (VP-ODS, 5 μm, 4.6 mm × 150 mm, Shimadzu, Kyoto, Japan) with the isocratic elution of acetonitrile:methanol:2-propanol (55:40:5, v/v/v), flow rate 1.0 mL/min, column oven at 30 °C (CO-965, JASCO, Tokyo, Japan), and the UV detector set at 325 nm (Tosoh, UV-8020, Tokyo, Japan). An aliquot of 20 μL for each sample dissolved in ethanol was applied to the HPLC column. The SF amount in the grain was quantitatively determined referring to the analysis of oryzanol standard. For calibration curve, the oryzanol standard solutions of 0.5, 1.0, 2.0, and 3.0 μg were analyzed under the same HPLC condition. Each calibration point was done in triplicate. The amount of SF in the grain was estimated by the calibration curve which was based on total peak area of the oryzanol standard. Therefore, the unit of SF amount extracted from the different grains was expressed in terms of “mg-oryzanol-equivalent.”
Identification of SF components by APCI-MS
SF components from whole wheat grain (Yumekaori) were characterized by HPLC retention time and negative ion (NI) spectra from APCI-MS as described earlier by Hakala et al.Citation19) with minor modifications. Samples were collected by the fractionation of each HPLC peak under the HPLC analysis conditions described above. The SFs in the three fractions were analyzed using a LTQ-Orbitrap velos mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Mass spectrometric analysis was conducted using an APCI source in NI mode. Mass calibration was performed daily using an ESI NI calibration solution (Thermo Fisher Scientific) and polytyrosine (Pierce® Triple quadrupole calibration solution, Thermo Fisher Scientific). The fractioned samples were dissolved in 50% methanol solution (methanol:H2O, 50:50, v/v) and applied to the ion source using a direct flow injection technique. The vaporizer temperature and capillary gas were set at 300 and 250 °C, respectively. The data were acquired using a Fourier transform full scan with a resolution power of 30,000 and the microscan count fixed to unity. The automatic gain control was set at 1 × 106 and the mass spectrometric data were collected in a range of m/z value from 100 to 1000. Collision-induced dissociation (CID) was produced with helium (99.96%) at a pressure of 10−6 mTorr in the ion trap. Mass chromatograms and spectral data were acquired with Xcalibur software 2.0 (Thermo Scientific).
Results and discussion
SF contents in whole wheat grains
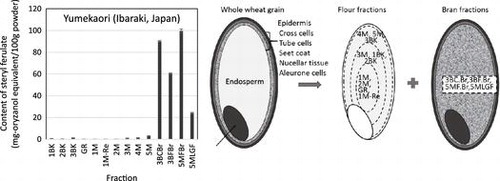
The SFs extracted from whole wheat grain, brown rice, and flour were quantitatively analyzed using HPLC, as shown in Fig. . The total SF per 100-g grain or flour were as follows: 35.7 ± 1.3 mg-oryzanol-equivalent in Koshihikari (brown rice), 15.2 ± 1.3 mg-oryzanol-equivalent in Yumekaori (whole wheat grain), 15.4 ± 1.1 mg-oryzanol-equivalent in Kitahonami (whole wheat grain), 11.4 ± 1.3 mg-oryzanol-equivalent in 1CW (whole wheat grain), 13.5 ± 1.2 mg-oryzanol-equivalent in DNS (whole wheat grain), 0.1 ± 0.1 mg-oryzanol-equivalent in Kameriya (flour), and 0.1 ± 0.1 mg-oryzanol-equivalent in Savory (flour), respectively. In addition, the total contents of SF in other whole wheat varieties produced in Japan were likewise evaluated by HPLC analysis. The SF contents of Haruyutaka, Haruyokoi, Harukirari, Hanamanten, Satonosora, Kitanokaori, Yumechikara and Ayahikari were ranged from 9.5 ± 0.5 to 17.2 ± 1.3 mg-oryzanol-equivalent/100 g grain, respectively. On the other hand, the SF contents of other imported whole wheat varieties, SH, WW, ASW, and PH were from 11.2 ± 0.9 to 13.9 ± 0.8 mg-oryzanol equivalent/100g grain.
Fig. 1. HPLC chromatograms of brown rice (A), whole wheat grains (B–E), and commercially available flours (F, G). The oryzanol standard reagent was used to identify each peak. 1. Cycloartenyl ferulate, 2. 24-methylene cycloartanyl ferulate, 3. Campesteryl ferulate, 4. △7-sitosteryl ferulate, 5. β-sitosteryl ferulate, 6. Campestanyl ferulate, and 7. Sitostanyl ferulate (Stigmastanyl ferulate).
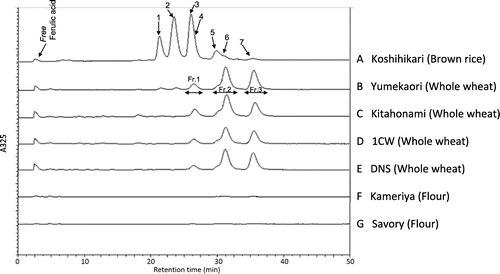
For the qualitative analysis of SFs, a typical HPLC pattern for SF components of brown rice (oryzanol) is presented in Fig. and was in accordance with the finding of the previous study.Citation20) For the whole wheat grains, different HPLC patterns emerged compared to that found in brown rice (Fig. ). This suggests that the composition of SF components in the examined whole wheat grain was different from that in brown rice, oryzanol. Furthermore, several differences in HPLC patterns of SFs were found among the whole wheat grains (Yumekaori, Kitahonami, 1CW, and DNS), suggesting that there were small differences in the content and composition of SF components in the examined whole wheat varieties. The HPLC analysis indicated that very few SFs were present in the commercially available flours, Kameriya and Savory. Because of considerable differences in the HPLC pattern of SF between whole wheat grain and brown rice, the SF components of whole wheat grain should be further investigated for their qualitative characterizations. To this end, three main HPLC peaks of SF from a whole wheat grain (Yumekaori) were fractionated for the identification of the components using APCI-MS method.
Identification of SF components of Yumekaori
The identification of an individual SF component was performed on the basis of the relative retention times of the HPLC and of APCI-MS analyses. The isolated HPLC fractions from a whole wheat grain of Yumekaori were designated Fr.1, Fr.2, and Fr.3 (Fig. ). Each fraction was analyzed using the NI mode of APCI-MS. The specific detection of each SF component was possible due to the characteristic deprotonated molecule (M-H)- that was formed from each component (Fig. ). Automated MS/MS and MS/MS/MS programs were employed to get the product ion spectra from CID of base peaks in mass spectra. The results were completely good accordance with the previous study,Citation6) which was summarized in Table . Based on the NI mode APCI-MS analysis, the first eluting fraction of Yumekaori, Fr.1, contained two SF components whose deprotonated molecules (M-H)- were m/z 575.4 and m/z 589.4, respectively, with the former being the dominant component. Based on the previous studies,Citation19,21) the dominant component of Fr.1 was campesteryl ferulate (mw 576.4) and the minor component was △7-sitosteryl ferulate (mw 590.4). The NI mode APCI-MS analysis of Fr.2 included two co-eluted components, campestanyl ferulate (mw 578.4), and β-sitosteryl ferulate (mw 590.4) and the former was the main component in the Fr.2. The two kinds of sitosteryl ferulates in the Fr.1 and Fr.2 were distinguished based on previous HPLC chromatogram patterns.Citation19,21) The last eluted fraction, Fr.3, was composed of only one kind of SF component, sitostanyl ferulate, whose molecular weight was 592.5. As a result, campesteryl ferulate, campestanyl ferulate, and sitostanyl ferulate were the main SF components found in Yumekaori. This finding is in accordance with the results of previous studies on foreign wheat varieties.Citation19,21) In rice bran, the main components are cycloartenyl ferulate, 24-methylene cycloartenyl ferulate, campesteryl ferulate, and sitosteryl ferulate.Citation6) The results in this study reveals that the SF composition of the wheat produced in Japan, Yumekaori, is also considerably different from that of brown rice, Koshihikari, suggesting that the SF components of whole wheat grains would be useful for the comparative studies with those of oryzanol.
Fig. 2. Negative ion mode APCI-MS spectra of SF components in Fr.1, Fr.2 and Fr.3, which were prepared by the fractionation of the corresponding HPLC peaks of whole wheat grain Yumekaori.
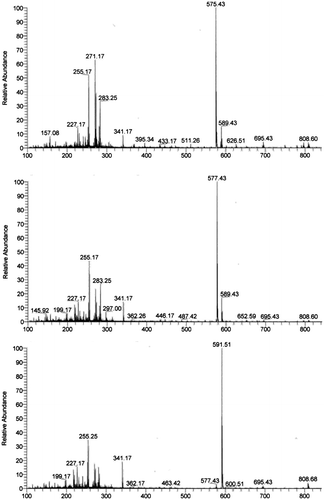
Table 1. APCI-MS data for sterol ferulates.
SF distribution in whole wheat grain
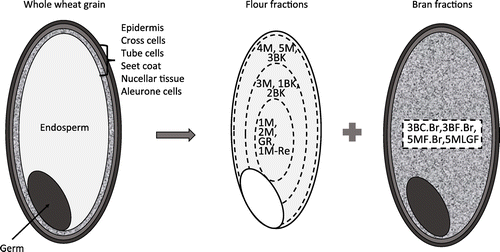
A recent study has shown that the higher the content of wheat bran is, the higher the SF is contained.Citation19) SFs are therefore thought to be distributed in certain layers of the wheat bran.Citation19) The knowledge of SF variation within the milled fractions of the whole wheat grain is necessary for the effective utilization of wheat’s SF. In this study, whole wheat grains of Yumekaori (Ibaraki, Japan) and 1CW (Canada) were separated into fourteen milling fractions using a Miag Laboratory Mill (test-mill). The fractionated conditions are summarized in Tables and . Ten fractions (1BK, 2BK, 3BK, GR, 1M, 1M-Re, 2, 3, 4, and 5) are mainly collected from endosperm as in flour milling. Among them, the fractions 1M, 2M, GR, and 1M-Re are the flour located near the center of endosperm, whereas 3M, 1BK, and 2BK are the fractions located in the intermediate between the center and the edge of the endosperm. The fractions 4M, 5M, and 3BK consist of the flour from the endosperm, which is located near the epidermis (Fig. ). On the other hand, the fractions 3BCBr, 3BFBr, 5MFBr, and 5MLGF contain the powder of wheat bran. The bran fraction from wheat milling corresponds to the germ and the peripheral part of the wheat kernel. The latter part is composed of multiple layers: epidermis, cross cells, tube cells, seet coat, nucellar tissue and aleurone layer. In the general milling streams, the peripheral layers and the germ cannot be completely separated from each other.Citation22,23) Therefore, it is difficult to elucidate the detailed SF localization in the peripheral and the germ form the results of the SF distribution in these four bran fractions.
Table 2. Condition of milling.
Table 3. Descriptions of wheat milling fractions.
Fig. 3. The model of the relation between structure of whole wheat grain and the localization of each fraction produced by a conventional test milling machine.
The HPLC analysis showed significant differences in SF amount among these 14 milling fractions. Most SFs were contained in the bran fractions 3BCBr, 3BFBr, 5MFBr, and 5MLGF. For example, the fractions, 3BCBr, 3BFBr, 5MFBr, and 5MLGF of Yumekaori contained SF at the level of 90.0 ± 2.8, 60.5 ± 1.5, 99.5 ± 2.8, and 24.0 ± 0.7 mg-oryzanol-equivalent/100 g fraction, respectively (Fig. ). The SF contents of the ten flour fractions of Yumekaori ranged from 0.2 to 2.4 mg-oryzanol-equivalent/100 g fraction (Fig. ).
Fig. 4. Total SF contents of the milling fractions of whole wheat grain 1CW (A) and Yumekaori (B). The milling processes using the test mill are summarized in Tables and . The flour fractions were 1BK, 2BK, 3BK, GR, 1M, 1M-Re, 2, 3, 4, and 5. In contrast, the bran fractions were 3BCBr, 3BFBr, 5MFBr, and 5MLGF.
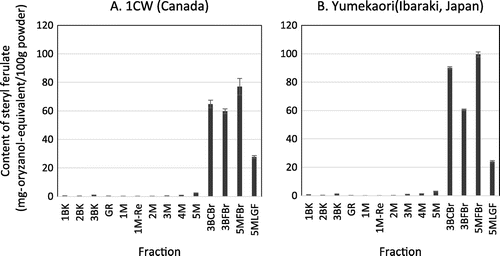
As mentioned above, the SF contents in whole wheat grains of Yumekaori and 1CW were 15.2 ± 1.4 and 11.4 ± 1.3 mg-oryzanol-equivalent/100 g grain, respectively. The SF content of the bran fractions of Yumekaori was much higher than that of the corresponding 1CW fraction (Fig. ). The bran fractions of Yumekaori contained SF in the range, from 24.0 to 99.5 mg-oryzanol-equivalent/100 g wheat bran, whose concentrations were about 1.6–6.6-fold of that of the whole wheat grain. In the case of brown rice, the SF levels in the bran fraction were 60–240 mg/100 g rice bran.Citation24,25) These results show that the wheat bran fractions produced in the milling could be an attractive and available SF source, like as the rice bran. The results in this study supported that those of the previous studies that the SFs were concentrated in the bran of the whole wheat grain.Citation26,27) However, the SF contents of bran samples changed considerably when the sampling was repeated and their variation depended on the grain raw materials, post-harvest processing, storage conditions, and milling processes.Citation19) Further information about SFs of whole wheat grains distributed in Japan should be accumulated.
Following wheat milling, only refined white endosperm-based flour is generally used for food products. In contrast, the bran fraction that contains most of the bioactive and potentially healthy components is dealt with a by-product that is discarded for animal feed. The present study suggested that most SF in whole wheat grain may be lost when certain flour fractions are produced in common flour-milling procedures. In actuality, the SF contents are fairly low, as shown in the analysis of the commercially available flour, Kameriya and Savory (Fig. ). However, as the final flour product is sometimes a mixture of several milling fractions, those with a high SF concentration may be added back to the final flour product with a permissible range of consumer acceptability. To enhance the SF intake from wheat, it is therefore important to specify the fractions with high SF concentrations among the milling fractions.
On the other hand, wholegrain products are rich in dietary fiber, vitamins, minerals, and phytochemicals and their further consumptions are recommended for human health benefits.Citation14) The results of this study also suggest that food products made from whole wheat grains, such as whole wheat grain breads, are good sources of SF. Increasing consumption of whole wheat products are related to the increase in the intake of SFs.
Especially in Japan, oryzanol, SF in rice bran, is popular and industrialized source of SF. However, SF in whole wheat grains should be noticed and introduced into various Japanese industrial usages.
Furthermore, investigations about SFs of wheat would be brought several alternatives as oryzanol-like functional factors to physiological studies, because of the differences in SF components between whole wheat grains and brown rice.
Author contribution
WT designed and performed experiments, analyzed data and wrote the paper. HM and SK (Kawahara) designed and performed experiments. EK and SK (Komba) designed experiments and SY analyzed data and wrote the paper. YK developed analytical methods. AH administered the experiment and wrote the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages.
Acknowledgments
The authors wish to thank Miss Tomoko Sato for an excellent APCI-MS technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Andersson AAM, Dimberg L, Åman P, et al. Recent findings on certain bioactive components in whole grain wheat and rye. J Cereal Sci. 2014;59:294–311.10.1016/j.jcs.2014.01.003
- Nyström L, Mäkinen M, Lampi A-M, et al. Antioxidant activity of steryl ferulate extracts from rye and wheat bran. J Agric Food Chem. 2005;53:2503–2510.10.1021/jf048051t
- Woyengo TA, Ramprasath VR, Jones PJH. Anticancer effects of phytosterols. Eur J Clin Nutr. 2009;63:813–820.10.1038/ejcn.2009.29
- Burlando B, Cornara L. Therapeutic properties of rice constituents and derivatives (Oryza sativa L.): a review update. Trends Food Sci Technol. 2014;40:82–98.10.1016/j.tifs.2014.08.002
- Kaneko R, Tsuchiya T. New compound in rice bran and germ oil. J Chem Soc Jpn. 1954;57:526.
- Fang N, Yu S, Badger TM. Characterization of triterpene alcohol and sterol ferulates in rice bran using LC-MS/MS. J Agric Food Chem. 2003;51:3260–3267.10.1021/jf021162c
- Rogers EJ, Rice SM, Nicolosi RJ, et al. Identification and quantitation of g-oryzanol components and simultaneous assessment of tocols in rice bran oil. J Am Oil Chem Soc. 1993;73:811–813.
- Xu Z, Godber JS. Purification and identification of components of γ-oryzanol in rice bran oil. J Agric Food Chem. 1999;47:2724–2728.10.1021/jf981175j
- Norton RA. Quantitation of steryl ferulate andp-coumarate esters from corn and rice. Lipids. 1995;30:269–274.10.1007/BF02537832
- Baranowski JD, Nagel CW. Inhibition of Psudomonas fluoresens by hydroxycinnamic acids and their alkyl esters. J Food Sci. 1982;47:1582–1589.
- Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23:65–134.10.1017/S0954422410000041
- Nurmi T, Lampi A-M, Nstrom L, et al. Disribution and composition of phytosterol and steryl ferulates in wheat grain and bran fractions. Cereal Sci. 2012;56:379–388.
- Berger A, Rein D, Schafer A, et al. Similar cholesterol–lowering properties of rice bran oil, with varied γ–oryzanol, in mildly hypercholesterolemic men. Eur J Nutr. 2005;44:163–173.10.1007/s00394-004-0508-9
- Nurmi T, Lampi A-M, Nyström L, et al. Effects of environment and genotype on phytosterols in wheat in the healthgrain diversity screen †. J Agric Food Chem. 2010;58:9314–9323.10.1021/jf100192t
- Seitz LM. Stanol and sterol esters of ferulic and p-coumaric acids in wheat, corn, rye, and triticale. J Agric Food Chem. 1989;37:662–667.10.1021/jf00087a019
- Ward JL, Poutanen K, Gebruers K, et al. The HEALTHGRAIN cereal diversity screen: concept, results, and prospects. J Agric Food Chem. 2008;56:9699–9709.10.1021/jf8009574
- Nyström L, Paasonen A, Lampi A-M, et al. Total plant sterols, steryl ferulates and steryl glycosides in milling fractions of wheat and rye. J Cereal Sci. 2007;45:106–115.10.1016/j.jcs.2006.08.003
- Esche R, Barnsteiner A, Scholz B, et al. Simultaneous analysis of free phytosterols/phytostanols and intact phytosteryl/phytostanyl fatty acid and phenolic acid esters in cereals. J Agric Food Chem. 2012;60:5330–5339.10.1021/jf300878h
- Hakala P, Lampi A-M, Ollilainen V, et al. Steryl phenolic acid esters in cereals and their milling fractions. J Agric Food Chem. 2002;50:5300–5307.10.1021/jf025637b
- Pestana-Bauer VR, Zambiazi RC, Mendonça CRB, et al. γ-oryzanol and tocopherol contents in residues of rice bran oil refining. Food Chem. 2012;134:1479–1483.10.1016/j.foodchem.2012.03.059
- Fang N, Yu S, Badger TM. Characterization of triterpene alcohol and sterol ferulates in rice bran using LC-MS/MS. J Agric Food Chem. 2003;51:3260–3267.10.1021/jf021162c
- Wheat Flour Milling. Wheat: the raw material. 2nd ed. In: Posner ES, Hibbs AN, editors. Bran: AACCI Publications; 1997. Chaper 1. p. 5. ISBN: 978-1-891127-40-3.
- Fang N, Yu S, Badger TM, et al. Characterization of triterpene alcohol and sterol ferulates in rice bran using LC-MS/MS. J Agric Food Chem. 2003;51:3260–3267.10.1016/j.jcs.2007.09.013
- Landberg R, Kamal-Eldin A, Salmenkallio-Marttila M, et al. Localization of alkylresorcinols in wheat, rye, and barley kernels. J Cereal Sci. 2008;48:401–406.10.1016/j.jcs.2007.09.013
- Aguilar-Garcia C, Gavino G, Baragaño-Mosqueda M, et al. Correlation of tocopherol, tocotrienol, γ-oryzanol and total polyphenol content in rice bran with different antioxidant capacity assays. Food Chem. 2007;102:1228–1232.10.1016/j.foodchem.2006.07.012
- Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23:65–134.10.1017/S0954422410000041
- Chotimarkorn C, Benjakul S, Silalai N. Antioxidant components and properties of five long-grained rice bran extracts from commercial available cultivars in Thailand. Food Chem. 2008;111:636–641.10.1016/j.foodchem.2008.04.031
