Abstract
We investigated the effects of Sarcodon aspratus, Agaricus bisporus, and Lentinula edodes aqueous extracts on the tenderization of bovine longissimus dorsi muscle. Meat quality and muscle protein degradation were examined as well. Beef chunks were marinated in distilled water (control), 5% S. aspratus (SA), 5% A. bisporus (AB), or 5% L. edodes (LE) extracts. SA was shown to have a higher enzymatic activity (p < 0.001) and water-holding capacity than LE (p < 0.01). SA and AB extracts exhibited lower shear force values compared with the control (p < 0.05). SA, AB, and LE showed superior muscle proteolytic effects compared with the control. SA demonstrated the ability to degrade myosin heavy chains and actin, which was not observed after AB and LE extract treatments. This suggests that SA extract may affect tenderization. Taken together, our results show that aqueous extract of S. aspratus affects the tenderness of the bovine longissimus dorsi muscle.
Graphical Abstract
Effects of mushroom extract on textural properties and muscle protein degradation of bovine longissimus dorsi muscle.
Mushrooms belong to Basidiomycetes, a group of Eumycetes, fungi. With the multitude of unique tastes and flavors, they have been used in the human diet for a long time, but also for the treatment and prevention of various diseases.Citation1,2) Recently, the interest in the effects of different types of mushrooms on human health has been increasing steadily, together with the interest in organic, low-calorie, and functional foods. There are approximately 20,000 types of mushrooms identified to date, and they contain various bioactive materials, as well as many nutrients, such as different types of carbohydrates, protein, minerals, vitamins, and nucleic acids.Citation2,3) Many studies have reported that bioactive materials isolated from mushrooms show different pharmacological effects, such as anticarcinogenic, antioxidative, antibacterial, and anti-mutation effects, and that they may reduce cholesterol.Citation4,5)
In addition to the nutritional value of mushrooms, various studies have been investigating different enzymes identified in the edible mushrooms, and it was shown that Sarcodon aspratus, Agaricus bisporus, and Lentinula edodes contain proteolytic enzymes.Citation6–8) In particular, S. aspratus has been used in traditional medicine for the treatment of indigestion caused by the consumption of meat, and Lee et al.Citation9) showed that this mushroom contains a number of proteolytic enzymes. Shin et al.Citation10) reported that S. aspratus may increase water-holding capacity (WHC) and myosin heavy chain (MHC) degradation, and lead to an increase in the tenderness of meats.
Meat tenderness is one of the sensory characteristics of meat, and it represents a significant factor for the evaluation of meat quality. Many studiesCitation11,12) investigated this issue, and the temperature control of conductor, pelvic bone suspension, electrical stimulation, and the addition of meat tenderizer enzymes extracted from tropical plants were developed.Citation13,14) Currently, proteolytic enzymes isolated from tropical fruits are mainly used as meat tenderizers,Citation13,14) since they are safe and convenient, but the enzymes from edible mushroom can be used as meat tenderizer as well. Although it is known that S. aspratus, A. bisporus, and L. edodes contain proteolytic enzymes, especially, S. aspratus is known for its superior proteolytic effect, the possibility of its utilization and development as a tenderizer for beef has been appraised.Citation3) However, there are few published studies describing whether the proteases contained in S. aspratus have an impact on meat tenderization.Citation10) Additionally, meat tenderization using proteolytic enzymes isolated from A. bisporus and L. edodes mushrooms have not been investigated.
Here, we studied the effects of edible mushrooms S. aspratus, A. bisporus, and L. edodes, containing proteolytic enzymes, on the quality of meat and the increase in meat tenderness. Moreover, we investigated the degree of myofibrillar protein degradation, which can affect the tenderness, and performed further sensory evaluations.
Materials and methods
Study materials
S. aspratus (frozen) was purchased at Gyeongdong market, Seoul, South Korea, A. bisporus and L. edodes (Korean products) were obtained from Shin-Sun Foods, and beef (frozen loin muscle; product of Australia) was purchased at a Korean market.
Sample production
The mushrooms were cut into. 0.3 cm sections, freeze-dried (FD-5518, Ilshin Lab Co., South Korea). Dry samples were ground in a food mixer (Hanil Co., South Korea), sieved through 30-μm mesh, pulverized, and stored at –2 °C until further analyses.
Enzyme solution extraction and proteolytic enzyme activity measurements. (i) Enzyme solution extraction. One gram of freeze-dried S. aspratus, A. bisporus, and L. edodes powder was mixed with 20 mL of distilled water, and placed in incubator, with shaking, for 30 min (37 °C, 140 rpm). Afterward, the sample was filtered through a cheesecloth, and the filtrate was centrifuged for 20 min at 4 °C and 3500 ×g. The supernatant was regarded as the solution containing the protease from S. aspratus, A. bisporus, and L. edodes.
(ii) Enzyme activity measurements. The activity of proteases was measured according to Korean Food Standards Codex (2005). Casein (0.6%) was dissolved in 0.2 M sodium phosphate buffer, and the substrate solution was adjusted to pH 7.0. This solution (5 mL) was mixed with 1 mL of coenzyme solutions, and incubated for 10 min at 37 °C. Afterward, 2 mL of 0.44 M trichloroacetic acid were added, and the reaction was stopped. Following the incubation in the water bath at 37 °C for 30 min, it was filtered (Whatman No. 40, W. and R. Balston Ltd., UK), and 1 mL of residual solution was mixed with 5 mL of 0.55 M sodium carbonate. Folin-Ciocalteu reagent (1 mL; Sigma–Aldrich) was added, the solution was incubated at 37 °C for 20 min, and the absorbance was measured at 660 nm (BioTek, New Jersey, USA). The amount of tyrosine in the post-reaction solution was calculated from tyrosine (L-Tyrosine; Sigma–Aldrich) standard curve (y = 1.5719x + 0.0205, r2 = 0.9957). For the enzymatic activity, one unit was defined as the production of 1 μmol of tyrosine per 1 g of sample in 1 min.
Treatment conditions
Frozen beef sample was cut into 3 × 3 × 3 cm sections and freeze-dried. Powdered S. aspratus, A. bisporus, and L. edodes were used to obtain 5% extracts, which were placed into the vacuum polyethylene bags, and incubated at 4 °C for 48 h, before further experiments. The control samples were incubated with the distilled water for 48 h. Meat quality was determined by measuring pH, color, WHC, loss on heating, and shear force.
Meat quality measurements. (i) pH measurement. Beef treated with enzyme extract was immersed in liquid nitrogen and powdered using waring blender (HGB150, Christison Ltd., UK), and 5 g of this powder was mixed with 20 mL of distilled water and homogenized (6000 rpm, 40 s; Ace Homogenizer AM-8, Nissei Co., Japan). Following the incubation at room temperature for 5 min, pH was measured using portable pH meter (290A, Orion Research Inc., USA).
(ii) Meat color determination. Using a chroma meter (CR-200 Minolta, Japan), lightness (L*), redness (a*), and yellowness (b*) of beef treated with enzyme extract was measured. Mean value was obtained from more than three different areas of the sample, using three different samples. Chromo meter was calibrated using L* value of 94.50, a* value of 0.3032, and b* value of 0.3193.
(iii) Loss on heating test. Beef treated with enzyme extract was cut and the samples of the same shape and weight (20.0 ± 0.5 g), were placed into vacuum polypropylene bags, and heated in the water bath at 85 °C, until the core temperature reached 75 °C. Afterward, the samples were cooled in slurry ice until reaching the equilibrium under cooling condition (4 °C). The surface water from the sample taken out from the polypropylene bag was absorbed using a blotting paper, and the weight of the samples was measured. The weight lost by heating was expressed in the percentages of the initial weight.
(iv) Water-holding capacity (WHC) measurements. The method used to measure WHC was based on the revised experimental method of Hamm and Deatherage.Citation15) The sample (10 g), following the treatment with the extracts, was mixed with 15 mL of 0.6 M sodium chloride and homogenized for 1 min (Daunce homogenizer 7727-40, Pyrex, USA). After the additional incubation at 4 °C for 15 min, the sample was homogenized again, centrifuged at 3000 × g, at 4 °C for 25 min, and the volume of the supernatant was measured.
(v) Shear force measurements. Beef treated with enzyme extract was cut into 10-mm thick sections, with the diameter of 50 mm, parallel to muscle fibers, and the force was measured when the sample was perpendicular to the muscle fibers, with Warner-Bratzler Shear installed on Instron (Series IX, Instron Corp., USA). Chart speed was 120 mm/min, measured speed was 20 mm, and maximum load was 10 kg.
SDS-PAGE analysis of muscle protein. (i) Myofibrillar protein extraction. Samples required for protein extraction were immersed in liquid nitrogen for safety, and stored at –80 °C before further experiments. Talmage and RoyCitation16) method was used for protein extraction, while the extracted proteins were analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the modified Laemmli method.Citation17)
The samples were powdered using the waring blender (HGB150, Christison Ltd., England), and 100 mg of the samples were dissolved in 400 μL of homogenization buffer (250 mM sucrose, 100 mM KCl, 5 mM EDTA, 20 mM Tris, pH 6.8) and centrifuged (10,000 ×g, 4 °C, 10 min). This process was repeated two more times. After the centrifugation, the precipitate was dissolved in 400 μL of wash buffer (175 mM KCl, 2 mM EDTA, 0.54% Triton X-100, 20 mM Tris, pH 6.8) and centrifuged (10,000 ×g, 4 °C, 10 min). The precipitate, after supernatant was removed, was dissolved in 400 μL of the resuspension buffer (150 mM KCl, 20 mM Tris, pH 7.0), homogenized, and stored at low temperature. The entire process of protein extraction was performed at 4 °C.
To each obtained sample, 300 μL of sample buffer (10% β-mercaptoethanol, 4% sodium dodecyl sulfate, 125 mM Tris-HCl pH 6.8) was aliquoted, and the final concentration of proteins was adjusted to 2.00–2.50 μg/μL, using the burette method.Citation18) The samples were homogenized by a vortex mixer, and heated in the boiling water for 3 min.
(ii) Myofibrillar protein analysis. For myofibrillar protein degradation analysis, samples were analyzed using 10% SDS-PAGE (SE 260 Vertical Electrophoresis System, Hoefer Pharmacia Biotech Inc., USA). The total protein samples (10 μg/well) were loaded, and the upper running buffer (0.1 M Tris, 0.15 M glycine, 0.15% SDS) was different from the lower running buffer (0.025 M Tris, 0.2 M glycine, 0.1% SDS), according to the revised method of Laemmli.Citation17) Electrophoresis was performed at 4 °C and 40 mA current for 2 h.Citation19) The gels were stained using 0.05% Coomassie Blue R-250 (w/v), 40% methanol, and 7% acetic acid, at room temperature for 2 h, and decolored twice using 40% methanol and 7% acetic acid. Gel images were obtained using Kodak DC290 (Eastman Kodak Company, USA).
Texture characteristics
Beef treated with enzyme extract was incubated with the extracts for 48 h, placed in a vacuum polypropylene bag, and heated in the water bath at 85 °C, until the core temperature reached 75 °C. Afterward, the sample was taken from water bath, cooled down to the room temperature, and measured using texture analyzer (TA-plus, Lloyd Instruments Ltd., UK). Hardness, cohesiveness, springiness, adhesiveness, gumminess, and chewiness were measured three times in each sample and analyzed. The measured data were analyzed using NEXYGEN Plus Material Test and Data Analysis Software (Lloyd Instruments Co Ltd., UK).
Sensory quality evaluation
Sensory quality evaluation was performed in order to investigate the difference in the sensory quality of beef treated with edible mushroom extracts. Twelve graduate students were recruited as sensory quality evaluators and trained according to the American Meat Science Association (AMSA, 1995) method for 2 weeks. The beef samples immersed in the extracts for 48 h were placed into a vacuum polypropylene bag, and heated at 85 °C until the core temperature reached 75 °C. Afterward, the surface water of the samples was removed by blotting paper, and the samples were cut into 1 × 1 × 2 cm3 sections, before provided in a warm condition. The participants were asked to record color, flavor, juiciness, tenderness, and overall acceptability scores, which can reflect the sensory quality of the samples. The scoring system for the quality evaluation included color (1: dislike very much, 5: neither like nor dislike, 9: like very much), flavor (1: very weak, 5: neither strong nor weak, 9: very strong), juiciness (1: very dry, 5: neither moist nor dry, 9: very moist), tenderness (1: very tough, 5: neither tough nor tender, 9: very tender), and overall acceptance (1: dislike very much, 5: neither like nor dislike, 9: like very much) evaluation, based on a 9-point scale.
Statistical analysis. The results from each experiment were statistically analyzed using SPSS 12.0 software. Statistical significance was determined using ANOVA and Duncan’s multiple-range test, and the results were considered significant if p-value was <0.05.
Results and discussion
Enzymatic activity
The enzymatic activity of S. aspratus extract was shown to be 0.594 U/mL, which was significantly higher compared with the other investigated extracts (p < 0.001), and it was 3.52 and 4.21 times higher than the activity of A. bisporus (0.160 U/mL) and L. edodes (0.141 U/mL) extracts, respectively (Table ). Eun et al.Citation20) showed that when the protein digestibility of edible mushrooms, such as S. aspratus, L. edodes, pine mushroom, ear mushroom, and rock mushroom, were analyzed, S. aspratus had three times higher protein digestibility than L. edodes, and four times higher than ear mushroom at pH 4.5. Additionally, at pH 7.0, the protein digestibility of S. aspratus was approximately 23 times higher compared with the activity of L. edodes, and 2.5 and 315 times higher compared with the activities of pine and ear mushrooms, respectively. Similar to these results, the proteolytic activity of S. aspratus was shown to be considerably higher than the activity of other types of edible mushrooms,Citation21) which agrees with the results obtained in this study. Furthermore, it was reported that the treatment of food proteins with the enzyme fraction of S. aspratus showed almost the same effects when the standard pepsin was applied.Citation22) Therefore, S. aspratus extracts should lead to an increase in meat tenderness.
Table 1. Proteolytic enzyme activities after freeze drying of Sarcodon aspratus, Agaricus bisporus and Lentinula edodes.
Meat quality measurements
pH measurements
The pH of the control sample, treated with distilled water, was shown to be 5.54, while the pH of the samples treated with S. aspratus, A. bisporus, and L. edodes extracts was shown to be 5.57, 5.60, and 5.55, respectively (Table ). In general, the pH of mushrooms is 6.0–6.7, and this can change depending on the soil or the growth conditions, but the results obtained in our experiments demonstrated that the addition of edible mushroom extracts has very little effect on the pH of beef.
Table 2. Meat quality traits of the bovine longissimus dorsi muscle treated with 5% Sarcodon aspratus (SA), 5% Agaricus bisporus (AB), 5% Lentinula edodes (LE).
Color
Color measurement results obtained using untreated and treated beef samples, are shown in Table . A significant difference in the lightness of meat (p < 0.001) between the control and mushroom extract-treated samples was demonstrated, and it was highest in the control sample (53.23), but low in the mushroom extract-treated samples (Table ). Redness of the control sample was determined to be 10.34, which was significantly (p < 0.001) lower compared with the redness of the mushroom extract-treated samples, but no significant difference was observed between the treated samples. Yellowness of S. aspratus-treated sample was determined to be 10.33, which was significantly higher than the yellowness of control (6.18, p < 0.001), A. bisporus- and L. edodes-treated samples (8.89 and 6.49, respectively, p < 0.001).
The changes in the meat color are caused by various factors, such as the pH of storage, maturation, temperature, lipid oxidation, and microbial growth.Citation23) The significant increase in the redness of the samples treated with the mushroom extracts, in comparison with the control sample, most likely reflects the color of the mushrooms themselves.
Loss on heating and WHC
Loss on heating results from the shortening of sarcomeres and the contractions of the muscle fibers when meat is heated.Citation24) L. edodes-treated sample was shown to lose 26.61% of the initial weight, demonstrating the lowest level of weight loss among all samples. A. bisporus-treated sample showed 31.55% of weight loss, S. aspratus-treated sample showed 35.32% of weight loss, while the control group showed 28.84% of the weight loss. No significant difference was observed between these three groups (Table ).
WHC describes the ability of meat to maintain the water already inside the meat, or the water added to the meat, throughout the processing of meat by cutting, heating, grinding, and compression.Citation25) WHC influences the tenderness of meat more than the entire water content, which is generally around 75%.Citation25,26)
Here, we determined that the WHC of S. aspratus-treated sample was 8.30%, which was significantly higher (p < 0.001) than the WHC of the control sample (6.58%), A. bisporus-treated sample (6.66%), and L. edodes-treated sample (5.12%). Becker et al.Citation27) reported that fiber shrinkage during heating reduced the WHC and decrease juiciness of meat. Similar to these results, the heating loss (%) and WHC (%) of sample showed similar trend. But there is no correlation between heating loss (%) and juiciness of meat in this study. Also, the cooking loss percentages of all treated samples significantly increased when compared with those of control samples accompanied with significantly lower values for fat retention for treated formulas.Citation28)
Our results agree with the results of a previous study conducted by Shin et al.Citation10) Additionally, Lee et al.Citation21) demonstrated that the addition of S. aspratus extract, kiwi, and pear, which increase tenderness and have proteolytic activities, increases the amount of water-soluble components by protein hydrolysis. Since the addition of mushroom extracts containing proteolytic enzymes affects WHC of the beef, this indicates that these extracts may lead to an increase in beef tenderness.
Texture
Table shows the results of the determination of the texture characteristics of beef samples treated with S. aspratus, A. bisporus, and L. edodes extracts. The determination of the hardness of meat samples can show the effects of the treatments on the increase in meat tenderness. The hardness of the S. aspratus-treated sample was shown to be 24.09 kgf, A. bisporus-treated sample was 28.83 kgf, and L. edodes-treated sample 31.56 kgf, which was significantly lower than the hardness of the control sample, determined to be 41.03 kgf (p < 0.001). The gumminess of S. aspratus-treated sample was significantly lower than the gumminess of the control sample as well (p < 0.001). Kim et al.Citation29) showed that the parameter that represents the most important indicator of the meant tenderness is gumminess, and hardness and gumminess of meat were shown to decrease following the incubation of meat with fruits containing proteolytic enzymes, which agrees with the results obtained in this study, suggesting that the proteolytic enzymes in mushroom extracts were able to degrade beef proteins.
Table 3. Shear force and textural properties of the bovine longissimus dorsi muscle with 5% Sarcodon aspratus (SA), 5% Agaricus bisporus (AB), 5% Lentinula edodes (LE).
Shear force
Shear force measurement results, highly associated with the tenderness of beef, are shown in Table . The shear force of S. aspratus-treated sample was 23.93, which was significantly lower compared with A. bisporus-treated (28.18) and L. edodes-treated samples (32.67) (p < 0.001). The shear force of the control sample was determined to be 45.07, significantly higher compared with the mushroom-treated samples (p < 0.001). The activity of the proteolytic enzymes in S. aspratus, A. bisporus, and L. edodes extracts may have affected these results, and we observed that the higher values of enzyme activity (Table ) of an extract correlated with the lower shear force values obtained following the sample treatments.
Shear force is the force applied during the mechanical cutting of meat, and low shear force meat is considered tenderized meat.Citation6) A previous study showed that a treatment of meat with fruit extracts containing a high amount of proteolytic enzymes leads to a decrease in the shear force.Citation2) Therefore, our results show that S. aspratus-treated meat samples have an increased tenderness, in comparison with other samples.
Myofibrillar protein analysis and SDS–PAGE
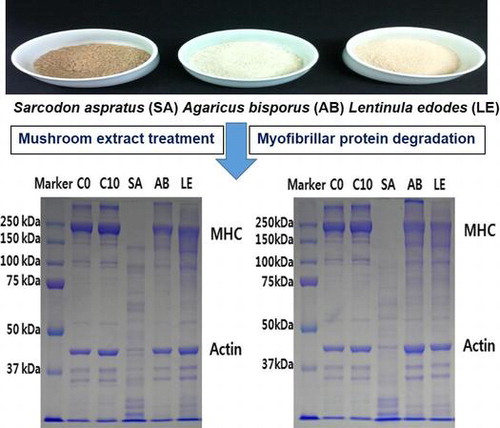
The changes in MHC and actin were observed 6, 24, and 48 h during the treatment of meat samples with the mushroom extracts (Fig. ). Six hours after the treatments, SDS-PAGE analyses showed that S. aspratus extract led to a higher rate of degradation of MHC and actin, in comparison with A. bisporus- and L. edodes-treated samples. Some degradation of MHC was observed in these two samples as well, but not actin degradation.
Fig. 1. Change in myosin heavy chain and actin in SDS–PAGE profile of the myofibrils prepared from Freeze drying mushroom powder 6 h (A) or 24 h (B) treated a slice of the bovine longissimus dorsi muscle. C0, control was the bovine longissimus dorsi muscle before treated; C10, control was the bovine longissimus dorsi muscle treated only distilled water; SA, the bovine longissimus dorsi muscle treated by freeze drying Sarcodon aspratus powder 5% (w/v); AB, the bovine longissimus dorsi muscle treated by freeze drying Agaricus bisporus powder 5% (w/v); LE, the bovine longissimus dorsi muscle treated by freeze drying Lentinula edodes powder 5% (w/v).
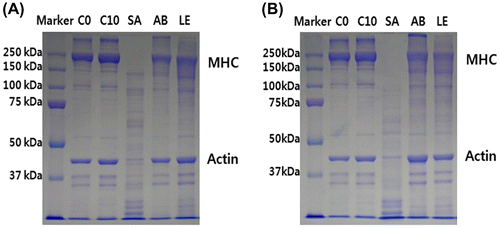
Furthermore, 24 h later, S. aspratus extract treatment led to a complete degradation of MHC, while an increased rate of actin degradation was observed as well. Additionally, in the samples treated with A. bisporus and L. edodes extracts, a higher rate of MHC degradation was observed, but actin molecules were still not degraded, demonstrating that the extracts of these two mushrooms are not able to degrade actin, but they do degrade MHC. No degradation was observed in the control sample in both time points.
The proteolytic enzymes in muscles, calpain, and cathepsin, lead to the fragmentation of myofibrillar proteins, but they rarely degrade myosin and actin.Citation30) Kim et al.Citation3) showed that the proteolytic enzymes of S. aspratus are able to degrade myofibrillar proteins, including MHC, and are able to increase meat tenderness, which agrees with the results obtained in this study.
Sensory quality evaluation
The results of sensory quality evaluation of beef samples treated with the edible mushroom extracts are presented in Table . The color of S. aspratus-, A. bisporus-, and L. edodes-treated samples was evaluated as 5.22, 5.81, and 6.70, respectively. The red color of cooked meat is mainly determined by the amount of myoglobin, its redox status, and the heat-dependent denaturation.Citation31) As the idiopathic color of S. aspratus has been reflected, it is understood that redness (a*) of sample group S. aspratus was the lowest.Citation3) Therefore, idiopathic color of S. aspratus may be attributed to the dislike color. The flavor of A. bisporus-treated sample was rated 6.09, S. aspratus-treated was rated 6.00, and L. edodes-treated sample was rated 5.70, which was significantly higher than the flavor score of the control group, which was 3.60 (p < 0.001). No significant difference was observed between the mushroom-treated samples, showing that the mushroom treatment in general improved the flavor of the meat. There was no significant difference in juiciness between the control and mushroom extract-treated samples. The tenderness of the sample treated with S. aspratus was rated 7.33, while the tenderness of A. bisporus-treated sample was rated 6.72, which was significantly higher than the tenderness score of L. edodes-treated sample and the control sample (p < 0.001). S. aspratus-treated meat was rated 6.33 for overall preference, which was significantly higher compared with the control group (4.80; p < 0.01). No significant differences in overall preference were observed between the mushroom extract-treated samples.
Table 4. Sensory evaluation scores of the bovine longissimus dorsi muscle with 5% Sarcodon aspratus (SA), 5% Agaricus bisporus (AB), 5% Lentinula edodes (LE).
The tenderness of meat is considered the most important factor affecting the choice of meat.Citation4) We showed that S. aspratus-treated and A. bisporus-treated samples both had high tenderness and overall preference scores, which suggests that these two parameters correlate. Additionally, sensory quality evaluation led to the same conclusions about the treatment with extracts obtained from different mushrooms as the results obtained in enzymatic activity, WHC, texture, shear force, and myofibrillar protein degradation analyses.
Conclusion
Here, we analyzed the effects of mushroom extracts containing proteolytic enzymes on the beef tenderness by evaluating enzymatic activity, their ability to degrade meat proteins, and sensory quality, and analyzing shear force and texture characteristics.
S. aspratus extract showed the highest activity among all investigated mushrooms. The extracts showed no effect on the pH of beef, while S. aspratus-treated meat was shown to have the highest WHC. Additionally, shear force, hardness, and gumminess, which are associated with the tenderness of meat, were analyzed, and S. aspratus-treated meat was shown to have the best characteristic, although L. edodes and A. bisporus extracts were shown to increase the tenderness of meat as well, compared with the control. All three mushroom extracts were able to degrade MHC molecules, while S. aspratus extracts was able to degrade actin as well. Sensory quality of different samples was evaluated, and the mushroom-treated beef samples were rated higher in terms of flavor, tenderness, and overall preference, but not in the terms of color and juiciness. S. aspratus-treated meat received the highest score in terms of tenderness and overall acceptance.
Therefore, the use of S. aspratus extract, containing a high amount of proteolytic enzymes, represents the most effective treatment for the increase in proteolysis and, consequently, beef tenderness, and the results of this study may help in the development of processed meat products.
Author contribution
Kyung-Ha Lee and Ho-Kyoung Kim performed analysis on all samples, interpreted data, wrote manuscript and equally contributed and should be regarded as co-first authors. Sae-Hun Kim, Kyoung-Hwan Kim, Young-Min Choi and Hyun-Hee Jin helped in data interpretation and manuscript evaluation. Seung-Joo Lee and Youn-Chul Ryu supervised development of work and acted as corresponding author.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the 2016 scientific promotion program funded by Dong-Eui Institute of Technology (DIT); the Research Center for Production Management and Technical Development for High Quality Livestock Products through Agriculture, Food and Rural Affairs Research Center Support Program, Ministry of Agriculture, Food and Rural Affairs [grant number 715003-07].
Acknowledgment
We are grateful to Sustainable Agriculture Research Institute (SARI) in Jeju National University for providing the experimental facilities.
References
- Wasser SP. The fungus among us. Eretz Magazine. 1997;1:52–69.
- Kim JS, Han JS, Lee JS. A survey on mushroom uses. Korean J Food Sci. 1994;10:291–295.
- Kim HK, Lee SH, Ryu YC. Tenderization of bovine longissimus dorsi muscle using aqueous extract from Sarcodon aspratus. Korean J Food Sci Anim Resour. 2015;35:533–540.10.5851/kosfa.2015.35.4.533
- Cheung LM, Cheung PCK, Ooi VEC. Antioxidant activity and total phenolics of mushroom extracts. Food Chem. 2003;80:1–7.
- Lee GD, Chang HG, Kim HK. Antioxidative and nitrite-scavenging activities of edible mushroom. Korean J Food Sci Technol. 1997;29:432–436.
- El-Ialaki ME, Hamza MA. Edible mushrooms as producers of amylases. Food Chem. 1979;4:203–211.
- Eun GS, Yang JH, Cho DY, et al. Studies on higher fungi in Korea (II) proteolytic enzyme of Agaricus bisporus (Lange) Sing. J Korean Pharm Sci. 1989;19:9–14.
- Eun JS, Yang JH, Cho DY, et al. Studies on higher fungi in Korea (I) activity of proteolytic enzyme from Sarcodon aspratus (Berk) S. Ito. J Pharm Invest. 1989;18:125–131.
- Lee TK. Purification and some characteristics of the proteolytic enzyme in fruitbody of Neungee [Sarcodon aspratus (Berk.) S. Ito]. J Korean Soc Food Sci Nutr. 1986;15:276–285.
- Shin HG, Choi YM, Kim HK, et al. Tenderization and fragmentation of myofibrillar proteins in bovine longissimus dorsi muscle using proteolytic extract from Sarcodon aspratus. J Korean Soc Food Sci Nutr. 2008;41:1389–1395.
- Koohmaraie M. Biochemical factors regulating the toughening and tenderization processes of meat. Meat Sci. 1996;43:193–201.10.1016/0309-1740(96)00065-4
- Hwang IH, Devine CE, Hopkins DL. The biochemical and physical effects of electrical stimulation on beef and sheep meat tenderness. A review. Meat Sci. 2003;65:677–691.10.1016/S0309-1740(02)00271-1
- Kang CK, Rice EE. Degradation of various meat fraction by tenderizing enzyme. J Food Sci. 1970;35:563–565.10.1111/jfds.1970.35.issue-5
- Yamasaki Y, Suzuki Y. Purification and properties of β-glucosidase and glucoamylase from Lentinus edodes. Agric Biol Chem. 1978;42:971–980.
- Hamm R, Deatherage FE. Changes in hydration solubility and charges of muscle proteins during hearting of meat. J Food Sci. 1960;25:587–610.10.1111/jfds.1960.25.issue-5
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy chain isoforms. J Appl Physiol. 1993;75:2337–2340.
- Laemmli UK. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature. 1970;227:680–685.10.1038/227680a0
- Gornall AG, Bardawill CJ, David MM. Determination of serum-protein by means of the biuret reaction. J Biol Chem. 1949;177:751–766.
- Rivero JL, Talmadge LRJ, Edgerton VR. A sensitive electrophoretic method for the quantification of myosin heavy chain isoforms in horse skeletal muscle: histochemical and immunocytochemical verifications. Electrophoresis. 1997;18:1967–1972.10.1002/(ISSN)1522-2683
- Eun JS, Yang JH, Cho DY. Studies on higher fungi in Korea (I). J Korean Pharm Sci. 1988;18:125–131.
- Lee SA, Song YS, Cho JW, et al. Effect of the Sarcodon aspratus on the physicochemical and sensory properties of cooked beef. J Korean Soc Food Sci Nutr. 2001;30:266–272.
- Park WH. Studies on enzymes of the higher fungi of Korea (I)-Identification of protease in Sarcodon aspratus. Korean J Mycol. 1985;14:25–30.
- Mancini RA, Hunt MC. Current research in meat color. Meat Sci. 2005;71:100–121.10.1016/j.meatsci.2005.03.003
- Cho SH, Park BY, Kim JH, et al. Nutritional composition and physico-chemical meat quality properties of Korean Hanwoo Bull Beef. J Anim Sci Technol (Kor.). 2007;49:871–880.
- Warner RD, Kauffman RG, Russell RL. Quality attributes of major porcine muscles: a comparison with the longissimus lumborum. Meat Sci. 1993;33:359–372.10.1016/0309-1740(93)90007-5
- Warner RD, Kauffman RG, Greaser ML. Muscle protein changes post mortem in relation to pork quality traits. Meat Sci. 1997;45:339–352.10.1016/S0309-1740(96)00116-7
- Becker A, Boulaaba A, Pingen S, et al. Low temperature cooking of pork meat — physicochemical and sensory aspects. Meat Sci. 2016;118:82–88.10.1016/j.meatsci.2016.03.026
- Abdel-Naeem HHS, Mohamed HMH. Improving the physico-chemical and sensory characteristics of camel meat burger patties using ginger extract and papain. Meat Sci. 2016;118:52–60.10.1016/j.meatsci.2016.03.021
- Kim MH, Rho JH, Kim MJ. Proteolytic effect of fruit flesh and crude enzyme extract from fruits on myofibrillar protein. Korean J Food Cook Sci. 2010;26:323–329.
- Lindequist U, Niedermeyer THJ, Julich WD. The pharmacological potential of mushrooms. Evid Based Complement Altern Med. 2005;2:285–299.10.1093/ecam/neh107
- King NJ, Whyte R. Does it look cooked? A review of factors that influence cooked meat color. J Food Sci. 2006;71:R31–R40.10.1111/jfds.2006.71.issue-4
