Abstract
Chemical investigation of the roots of Pinus densiflora led to the isolation of two new triterpenoids, (24S)-3β-methoxy-24,25-epoxy-lanost-9(11)-ene (1) and 29-acetoxy-3α-methoxyserrat-14-en-21α-ol (2), together with three known serratene-type triterpenoids (3–5) and four known diterpenoids (6–9). Their structures were determined by spectroscopic analyses.
Key words:
Pinus densiflora (Japanese red pine, Pinaceae) is an evergreen coniferous plant, which is distributed in Japan, the Korean Peninsula and Northeast China. The tree grows up to 30 m tall, and its woods are used as building material and fuel. Its highly versatile pine resin is made into adhesives, varnishes and food additives. In addition, P. densiflora has been the subject of research on its chemical components, chemical ecology and biological activity.Citation1–9) On the other hand, although various triterpenoids have been isolated from the Pinaceae family,Citation10,11) there are no reports on P. densiflora. In addition, little has been studied hitherto on phytochemicals from its roots or their bioactivity.Citation12,13) Herein, we report on the isolation and structural determination of two new triterpenoids, 1 and 2, together with seven known terpenoids (3–9). All of the compounds were evaluated for inhibitory activity against cancer cells (HeLa, HL-60, srcts-NRK), the malarial parasite (Plasmodium falciparum), and other microbes (Staphylococcus aureus, Escherichia coli, Aspergillus fumigatus, Pyricularia oryzae and Candida albicans).
The roots of P. densiflora (30 trees, aged 2 years) were collected from Tsukuba, Ibaraki, Japan in June 2015. Lyophilized roots (166.0 g) were chopped and extracted three times with MeOH for 3 weeks at room temperature. After each filtrate was concentrated under reduced pressure, all extracts were combined to obtain a red-brown syrup (24.6 g). Part of the crude extract (2.7 g) was partitioned three times between n-hexane (300 mL) and 90% MeOH aqueous solution (300 mL). The hexane layer was concentrated in vacuo to obtain 588.6 mg of yellow-brown oily material. Most of the hexane layer (500.0 mg) was separated by silica gel flash column chromatography (hexane-ethyl acetate (EtOAc) 40:1–1:1 (v/v) and CHCl3-MeOH 5:1) to obtain 12 fractions (H1–12). Fraction H6 (2.7 mg), corresponding to a spot where the Rf value was 0.6 in hexane–EtOAc 10:1, was confirmed to be pure 1. Fraction H9 (30.0 mg) was again separated by silica gel flash column chromatography (CHCl3-MeOH 50:1–20:1), affording seven fractions (H9-1–7). After the separation of fraction H9-2 (9.0 mg, Rf 0.50–0.55 at CHCl3-MeOH 15:1) by repeated preparative-TLC and elution with hexane-EtOAc-AcOH 10:2:1, fraction H9-2-3 (3.0 mg, Rf 0.50–0.52 in hexane-EtOAc-AcOH 10:2:1) was further purified by preparative RP-HPLC with MeOH, yielding 2 (0.5 mg) and 3 (1.2 mg).
Compound 1 was isolated as a white powder with m.p. 153–155 °C. Its molecular formula was determined as C31H52O2 from HREIMS data at m/z 456.3967 (calculated for C31H52O2, 456.3971), indicating six degrees of unsaturation. While the 1H- and 13C-NMR spectral data for 1, as shown in Table , were similar to those for (24S)-3β-methoxy-lanost-9(11)-ene-24,25-diol reported from Taiwania flousiana,Citation14) there were two key differences between the two compounds in their side-chain data. Two conspicuous clues, an upfield shift for signals corresponding to oxymethine at C-24 (δH 3.28/ δC 79.6 → δH 2.67/ δC 65.0 for 1) and oxygenated carbon at C-25 (δC 73.2 → 58.1 for 1) and an increase in degree of unsaturation from 5° to 6°, indicated the existence of an epoxide at C-24–C-25 into 1, not a diol. Thus, the planar structure of 1 was established (Fig. (A)), which was confirmed in detail from the individual 1H–1H COSY and HMBC correlations (Fig. (B)). The relative stereochemistry of 1 was then determined from the key NOESY correlations (Fig. (C)) and interpretation of 1D-NMR spectra. The orientation of the oxymethine at C-3 was established to be α-axial on the basis of the coupling constant data of H-3 [dd, J = 11.5 Hz (ax-ax), 4.0 (ax-eq)], which was supported by the NOESY correlations of H-3/H-28, H-28/H-5, H-5/H-7α and H-7α/H-30. Furthermore, to elucidate the absolute stereochemistry at C-24, we adopted the method of Emmons et al. for 1.Citation15) By this method, the 24S and 24R epimeric pairs of lanosterol can be distinguished based on the difference of 13C-NMR chemical shift values in CDCl3. Because a set of data for 1 in the side-chain region of the 13C-NMR spectrum was in good agreement with that of 24S-epoxylanosterol acetate; C-20 (δR 36.2, δS 36.3 and δ1 36.0), C-21 (δR 18.7, δS 18.5 and δ1 18.3), C-22 (δR 32.5, δS 32.7 and δ1 32.9), C-23 (δR 25.6, δS 25.9 and δ1 26.0), C-24 (δR 64.7, δS 64.9 and δ1 65.0), C-25 (δR 58.4, δS 58.1 and δ1 58.2), C-26 (δR 24.9, δS 24.9 and δ1 25.0) and C-27 (δR 18.7, δS 18.6 and δ1 18.7),Citation15) the 24S of 1 was suggested. Therefore, the structure of 1 was elucidated as (24S)-3β-methoxy-24,25-epoxy-lanost-9(11)-ene (Fig. (A)).
Table 1. 1H-(500 MHz) and 13C-(125 MHz) NMR data for 1 and 2 in CDCl3 (δ in ppm).
Fig. 1. Isolated compounds from the root of P. densiflora (A) and key correlations of 1H-1H COSY, HMBC (B, D) and NOESY (C).
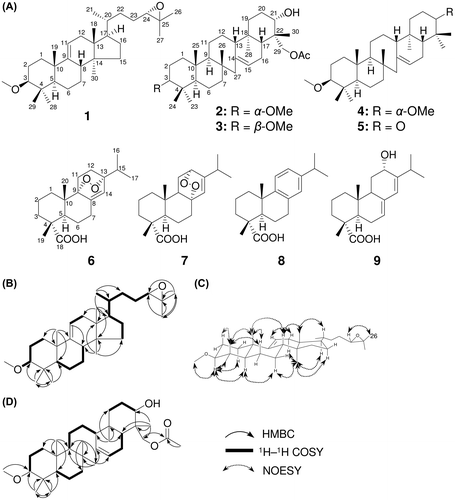
Compound 2 was obtained as a white powder (m.p. 216–218 °C). This compound exhibited a molecular ion peak [M]+ at m/z 514.4021 (calculated for 514.4022) in HREIMS, which indicates the molecular formula C33H54O4. The IR spectrum of 2 showed absorption bands at 3550 cm–1 (–OH) and 1716 cm–1 (C=O). The 1D- and 2D-NMR spectral data of 2 (Table , Fig. (D)) were in good agreement with those of 29-acetoxy-3β-methoxyserrat-14-en-21α-ol (3),Citation16) except for a methoxy methine signal at C-3 (δH 2.77/ δC 85.9 for 2, δH 2.62/ δC 88.4 for 3). The small coupling constants between H-3 and two adjacent H-2 protons (t, J = 2.5 Hz) in 2 indicated that H-3 took a β-equatorial orientation, which was confirmed by the NOESY correlations of H-3/H-24 and H-24/H-25. Therefore, the relative configuration of 2 was readily determined as 29-acetoxy-3α-methoxyserrat-14-en-21α-ol (Fig. (A)). The absolute stereo chemistry at C-21 remains to be determined by modified Mosher’s method because of very small amount of 1.
In addition to two new compounds, seven known terpenoids (3–9; Fig. (A)) were also isolated and identified by the analysis of spectroscopic data and comparison with previous reports.Citation17–22) To determine the bioactivity of the terpenoids, all compounds isolated (1–9) were evaluated for antiproliferative activity against malarial causing protozoa, cancer cell lines, bacteria and fungi (Supplemental materials).Citation23–24. Most compounds (1–3, 5–9) displayed antimalarial activity (IC50 values from 2.9 to 32 μM) (Table ); however, 4 did not. In addition, 8 exhibited a broad spectrum of antimicrobial activity against S. aureus, E. coli and P. oryzae. Compound 9 gave weak antibacterial activity against E. coli with an IC50 value of 28 μM.
Table 2. Bioactivity of isolated compounds 1–9 (IC50, μM).
In summary, we isolated two new triterpenoids, 1 and 2, together with seven other terpenoids, 3–9. Almost all of the terpenoids (1–3, 5–9) showed moderate antimalarial activity. Further detailed bioassays utilizing terpenoids would be required for orientation toward chemical biology research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Author contribution
J. O. and H. O. designed the experiments. The experiment, data analyses and interpretation were performed by J. O. Y. F. and J. O. performed bioassays. M. K. and Y. M. participated in the collection and lyophilization of plant materials. Manuscript was written by J. O., Y. F., and H. O.
Funding
This work was supported by the RIKEN SPDR Program (to J.O.), JSPS KAKENHI, and the Biology of Symbiosis Project of RIKEN.
Supplementary material
Supplemental material for this article can be accessed at http://dx.doi.org/10.1080/09168451.2016.1263149
Supplementary_File.pdf
Download PDF (747.1 KB)Acknowledgments
The authors are grateful to Dr. Y. Magae in FPRI for supplying the roots of Japanese red pine. We thank Dr. T. Nogawa in RIKEN for advice about spectrometric methods. We also thank Dr. T. Nakamura in RIKEN for the (HR) EI-MS and (HR) ESI-MS measurements. Our thanks are also due to Ms. H. Aono, Ms. M. Tanaka, Ms. M. Osada and Mr. K. Yamamoto in RIKEN for bioassays. This work was supported by the RIKEN SPDR Program (to J.O.), JSPS KAKENHI, and the Biology of Symbiosis Project of RIKEN.
References
- Kim YS, Shin DH. Volatile components and antibacterial effects of pine needle (Pinus densiflora S. and Z.) extracts. Food Microbiol. 2005;22:37–45.10.1016/j.fm.2004.05.002
- Kobayashi S, Ozawa T, Imagawa H. Dehydrochorismic acid from Pinus densiflora pollen. Agric Biol Chem. 1982;46:845–847.
- Jung MJ, Jung HA, Kang SS, et al. A new abietic acid-type diterpene glucoside from the needles of Pinus densiflora. Arch Pharm Res. 2009;32:1699–1704.10.1007/s12272-009-2206-x
- Kimura F, Sato M, Kato-Noguchi H. Allelopathy of pine litter: delivery of allelopathic substances into forest floor. J Plant Biol. 2015;58:61–67.10.1007/s12374-014-0322-8
- Herz W, Wahlborg HJ, Lloyd WD, et al. Resin acids. IV. 12-hydroxyabietic acid and its reduction. J Org Chem. 1965;30:3190–3195.10.1021/jo01020a078
- González MA, Pérez-Guaita D, Correa-Royero J, et al. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur J Med Chem. 2010;45:811–816.10.1016/j.ejmech.2009.10.010
- Gigante B, Santos C, Silva AM, et al. Catechols from abietic acid. Bioorg Med Chem. 2003;11:1631–1638.10.1016/S0968-0896(03)00063-4
- Savluchinske-Feio S, Curto MJ, Gigante B, et al. Antimicrobial activity of resin acid derivatives. Appl Microbiol Biotechnol. 2006;72:430–436.10.1007/s00253-006-0517-0
- Lekphrom R, Kanokmedhakul S, Kanokmedhakul K. Bioactive diterpenes from the aerial parts of Anisochilus harmandii. Planta Med. 2010;76:726–728.10.1055/s-0029-1240656
- Kukina TP, Shmidt EN. Triterpenoids inherent in the coniferous plants of Pinaceae family. Chem Sustain Dev. 2011;19:611–615.
- Tanaka R, Matsunaga S. Terpenoids and steroids from several Euphorbiaceae and Pinaceae plants. Yakugaku Zasshi (J Pharm Soc Jpn). 1999;119:319–339 (in Japanese).
- Joo CG, Lee KH, Park C, et al. Antioxidative activities and composition analysis of Pinus densiflora root by ultra high pressure extraction. J Indus Eng. Chem. 2011;17:712–716.10.1016/j.jiec.2011.05.018
- Watanabe T, Inaba K, Nakai A, et al. Water-soluble polysaccharides from the root of Pinus densiflora. Phytochemistry. 1991;30:1425–1429.10.1016/0031-9422(91)84178-U
- Xiang Y, Yang S, Zhan Z, et al. Terpenoids and phenols from Taiwania flousiana. Acta Bot Sin. 2004;46:1002–1008.
- Emmons GT, Wilson WK, Schroepfer GJ Jr. 24,25-epoxysterols. Differentiation of 24R and 24S epimers by 13C nuclear magnetic resonance spectroscopy. Phytochemistry. 1989;30:133–137.
- Rowe JW, Bower CL. Triterpenes of pine barks: naturally occurring derivatives of serratenediol. Tetrahedron Lett. 1965;6:2745–2750.10.1016/S0040-4039(01)83904-6
- Barrero AF, Sanchez JF, Alvarez-Manzaneda EJ, et al. Endoperoxide diterpenoids and other constituents from Abies marocana. Phytochemistry. 1991;30:593–597.10.1016/0031-9422(91)83732-Z
- Monaco P, Parrilli M, Previtera L. Two endoperoxide diterpenes from Elodea canadensis. Tetrahedron Lett. 1991;28:4609–4610.
- Kanno H, Schuller WH, Lawrence RV. Some reactions of levopimaric acid dioxide. J Org Chem. 1966;31:4138–4142.10.1021/jo01350a061
- Srivastava S, Kulshreshtha DK. Centdaroic acid, a new diterpene acid from Cedrus deodara. Indian J Chem. 2001;40B:348–349.
- Hayase H, Watanabe N, Lim CL, et al. Inhibition of malaria parasite growth by quinomycin A and its derivatives through DNA-intercalating activity. Biosci Biotechnol Biochem. 2015;79:633–635.10.1080/09168451.2014.987205
- Jang JP, Nogawa T, Uramoto M, et al. RK-270A-C, new oxindole derivatives isolated from a microbial metabolites fraction library of Streptomyces sp. RK85-270. J Antibiot. 2015;68:293–295.10.1038/ja.2014.141
- Wada S, Iida A, Tanaka R. Triterpene constituents from the stem bark of Pinus luchuensis and their DNA topoisomerase II inhibitory effect. Planta Med. 2001;67:659–664.10.1055/s-2001-17360
- Tanaka R, Mun C, Usami Y, et al. 3-Oxo-serratene triterpenoids from the stem bark of Picea jezoensis Carr. hondoensis. Phytochemistry. 1994;35:1517–1522.10.1016/S0031-9422(00)86888-0
