Abstract
Polyamines have beneficial properties to prevent aging-associated diseases. Raw soybean has relatively high polyamine contents; and the fermented soybean natto is a good source of polyamines. However, detailed information of diversity of polyamine content in raw soybean is lacking. The objectives of this study were to evaluate differences of polyamines among raw soybeans and select the high polyamine-containing cultivar for natto production. Polyamine contents were measured chromatographically in 16 samples of soybean, which showed high variation among soybeans as follows: 93–861 nmol/g putrescine, 1055–2306 nmol/g spermidine, and 177–578 nmol/g spermine. We then confirmed the high correlations of polyamine contents between raw soybean and natto (r = 0.96, 0.95, and 0.94 for putrescine, spermidine, and spermine, respectively). Furthermore, comparison of the polyamine contents among 9 Japanese cultivars showed that ‘Nakasen-nari’ has the highest polyamine contents, suggesting its suitability for enhancement of polyamine contents of natto.
Graphical abstract
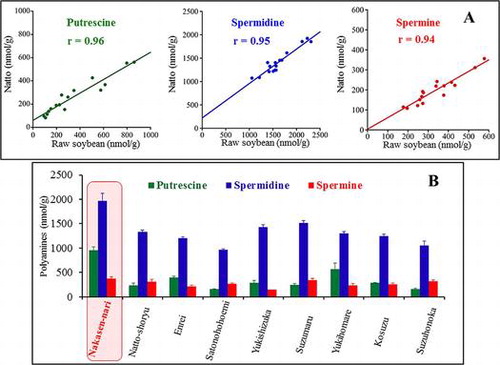
A. High correlations of polyamine contents between raw soybean and natto.
B. “Nakasen-nari” has the highest polyamine contents among 9 Japanese cultivars.
Polyamines are aliphatic polycations that are organic bases with important roles in the growth and function of normal cells.Citation1) The physiological polyamines such as putrescine (Put), spermidine (Spd), and/or spermine (Spm) are present in animals, plants, and micro-organisms.Citation2–4) They also contribute to the inhibition of age-associated global alterations in DNA methylation status, indicating that polyamine intake can help to prevent aging-associated diseases.Citation5)
Soybean (Glycine max L.) is a suitable crop for polyamine intake owing to its relatively high polyamine content. However, previous studies have shown that the polyamine contents of soy-based foods (soymilk, soybean curd, miso, and soy sauce) were lower than those of raw soybean, indicating that polyamines are reduced during the production processes.Citation6–9) In contrast, natto, a traditional fermented soy product in Japan, contains higher polyamines compared to other soy-based foods.
Natto is produced as follows:Citation10) (1) the raw soybean is washed and soaked in water; (2) after soaking, the soybeans are steamed using a pressure cooker; and (3) the steamed soybeans are fermented by Bacillus subtilis (natto). In a previous study, we monitored the polyamine variations during natto production, and found that the polyamine contents of natto were largely retained during the steaming processes and B. subtilis (natto) produced Spd during fermentation.Citation11) In addition, Toro-Funes et al.Citation12) indicated that natto showed the highest content of polyamines among several fermented and non-fermented products tested. In fact, the long-term consumption of natto by human volunteers has been reported to increase the blood Spm level.Citation13)
The polyamine contents of natto are supposed to depend on those of soybean, although this relationship has not been directly proven. Moreover, there is a general lack of information on the differences in polyamine levels among raw soybeans. Previous studies indicated variation in polyamines among different types of soybeans, with an Spd content ranging from 608 to 1430 nmol/g, and an Spm content ranging from 150 to 340 nmol/g.Citation6−Citation9) Similarly, Glória et al.Citation14) showed that polyamine contents varied according to both cultivar and harvest year in soybeans. These findings indicate the existence of a high polyamine-containing soybean cultivar, which may be useful for enhancing the polyamine contents of natto.
In this study, we examined the polyamine contents of soybeans distributed in Japan, and confirmed the relationship between polyamine contents of raw soybeans and natto. In addition, comparison of the polyamine contents among soybean cultivars was performed for selection of a high polyamine-containing cultivar.
Materials and methods
Plant materials and bacteria
Sixteen raw soybeans were used for the production of natto. ‘Suzuotome’ was kindly provided from the National Agricultural and Food Research Organization (NARO), Kyushu Okinawa Agricultural Research Center. The other samples were purchased from a local wholesaler. Each sample was harvested in 2012 or 2013 (Table ).
Table 1. Properties and the chemical compositions of soybean cultivars.
For selection of the highest polyamine-containing cultivar, 36 samples of 9 cultivars were collected. Each sample of one cultivar was different in harvest location, cultivation condition, or harvest years. Twenty-nine samples were kindly provided from the following agricultural experimental stations: 6 samples of ‘Nakasen-nari’, Nagano Vegetable and Ornamental Crops Experimental Station; 4 samples of ‘Enrei’, Niigata Agricultural Research Institute Crop Research Center; 4 samples of ‘Satonohohoemi’, 3 samples of ‘Kosuzu’, and 3 samples of ‘Suzuhonoka’, NARO Tohoku Agricultural Research Center; and 3 samples of ‘Yukishizuka’, 3 samples of ‘Suzumaru’, and 3 samples of ‘Yukihomare’, Hokkaido Research Organization, Agricultural Research Department. The other samples were purchased from a local wholesaler. Except for 1 sample harvested in 2013 of ‘Natto-shoryu’, all samples were harvested in 2014. Soybean seed was milled and stored at −40 °C until analysis.
B. subtilis (natto) was obtained from Miyagino-nattou Co., Ltd. (Miyagi, Japan). Before use, the spores were heated to inactive residual vegetative cells by suspending them in hot water (106 cells/mL) at approximately 80 °C.
Chemicals
Putrescine dihydrochloride, spermidine trihydrochloride, and spermine tetrahydrochloride were purchased from Sigma–Aldrich Co. (St. Louis, MO, USA). Diaminohexane dihydrochloride was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
Seed chemical compositions
Seed proteins were quantified by the Kjeldahl method. After addition of H2SO4 and KJELTABS®CT (FOSS, Hillerød, Denmark), sample was digested at 420 °C for about 3 h. Distillation and titration were carried out using Kjeltec™ 8400 (FOSS, Hillerød, Denmark). Nitrogen level was converted to protein level with a nitrogen factor of 5.71. Lipids were determined by Soxhlet extraction using diethyl ether as solvent. Ash was weighed after ash making at 650 °C for 8 h. Carbohydrate levels were calculated using the percentages of proteins, lipids, and ash as shown in Equation (1).(1)
Sucrose, raffinose, and stachyose contents were analyzed using high-performance liquid chromatography (HPLC) as follows: separation column, NH2P-40 2E (2.0 × 250 mm, Showa Denko K. K., Tokyo, Japan); eluent, CH3CN/H2O = 77.5/22.5; flow rate, 0.20 ml/min; column temperature, 40 °C; detector, ELSD-LTII (Shimadzu Co., Kyoto, Japan). The free sugar content was determined as the sum of sucrose, raffinose, and stachyose.
Production of natto
Soybean seeds were washed, soaked in water for 20 h at 15 °C, and steamed at 121 °C. Steaming time depended on the size of the seeds as follows: 40 min and 50 min for a 100-kernels weight of 8.2–14.2 g and 26.9–61.8 g, respectively. After heat-shocked spores of B. subtilis (natto) were inoculated into the steamed soybeans (inoculum rate: 10Citation4 cells/g), 45 g of the inoculated soybeans was placed in a polystyrene paper pack and transferred to a fermentation chamber. To control the microbial activity, soybeans were fermented under two different conditions as follows: a weaker activity condition at 39 °C under 90% relative humidity (RH) for 20 h, and a stronger activity condition at 42 °C under 95% RH for 22 h. After fermentation, natto products were dried at 20 °C and 50% RH for 2 h and subsequently chilled at 5 °C for 24 h. During fermentation, the product temperature was observed using a data logger (TR-71nw, Move Co., Tokyo, Japan) to monitor the microbial activity. Steamed soybeans and fermented products were freeze-dried, milled, and stored at −40 °C until the determination of polyamines.
Determination of polyamines
Polyamines were extracted from 1 g of the samples with a 5% trichloroacetic acid solution as previously described.Citation11) Before extraction, 1000 nmol of diaminohexane dihydrochloride was added to the sample as an internal standard.
Polyamines were analyzed by post-column chromatography using o-phthalaldehyde. Separation was performed with two tandem TSKgel polyaminepak columns (4.6 × 50 mm, Tosoh Co., Tokyo, Japan) according to the methods of Nishimura et al.Citation7) with a slight modification. The HPLC conditions were as follows: elution buffer: 93 mM sodium citrate, 2 M NaCl, 20% methanol, 0.08% Brij-35, and 0.64 mM N-caproic acid; regeneration solution: 0.8% NaOH; o-phthalaldehyde solution: 0.4 M boric acid, 0.35 M potassium hydroxide, 4 mM o-phthalaldehyde, 28 mM 2-mercaptoethanol, 0.1% Brij-35, and 0.63% methanol; flow rate: 0.42 ml/min; column temperature: 50 °C; program time: elution buffer for 55 min, regeneration buffer for 5 min, and elution buffer for 10 min. The fluorescence was measured at an excitation wavelength of 340 nm and an emission wavelength of 460 nm.
Statistical analysis
Data are expressed as means ± standard error of the mean (SE). The strength of correlation was evaluated according to the Pearson correlation coefficient (r). A p-value < 0.05 was considered to be statistically significant.
Results and discussion
Polyamines, properties, and chemical compositions of raw soybean
The compositions of different polyamines in raw soybeans and overall means and SEs are provided in Table . Each raw soybean displayed a different polyamine composition and, in particular, ‘Nakasen-nari’, ‘Suzumaru’, and ‘China’ contained higher polyamine levels than the others (Table ). The properties and chemical compositions of the 16 raw soybeans are also shown in Table , also showing high variation. These results suggested that each raw soybean has specific properties and chemical compositions, including polyamine contents.
To reveal the chemical profile of the high polyamine-containing soybeans, we analyzed the correlations between polyamine contents and chemical compositions as shown in Table . Put was positively correlated with sucrose (p = 0.00152) and free sugar (p = 0.00681). Spd and lipids exhibited a negative correlation (p = 0.00331), whereas Spd was positively correlated with carbohydrates (p = 0.00213), sucrose (p = 0.0424), and free sugar (p = 0.0176). By contrast, Spm was only significantly correlated with Spd (p = 0.0290). Thus, the correlation analysis showed that polyamines were related to the chemical compositions in soybeans, and, in particular, the carbohydrate and sugar contents could be used as indicators of polyamine contents. In contrast, there were no significant relationships between the polyamines and ash, although polyamines play an important role in abiotic stress tolerance such as high salinity in plants.Citation15)
Polyamine variations during natto production
We further investigated the changes in Put, Spd, and Spm contents during natto production, and the values in each soybean sample are shown in Table . The polyamine contents of steamed soybean and natto showed the high variations in polyamines similarly to raw soybean. In addition, fermentation condition (39 and 42 °C) did not affect the polyamine contents.
Table 2. Changes in polyamine contents during natto production.
Collectively, the Put content slightly reduced throughout the production process. The Spd content decreased during the steaming process and increased during the fermentation process, whereas the Spm content decreased throughout all processes. The observed reduction in polyamines during the steaming process was found to result from heat treatment, in accordance with a previous study.Citation16) Similarly, increases in the Spd content during fermentation process were reported previously.Citation10) On the other hand, reduction in Spm was presumed to be due to the degradation by spermidine/spermine acetyltransferase derived from bacteria.Citation17,18)
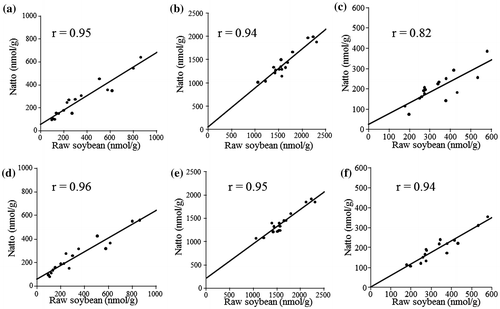
The correlations of each type of polyamine between raw soybean and natto were high under 39 °C (r > 0.82) and 42 °C (r > 0.94), as shown in Fig. . These data demonstrated the strong dependency of polyamine contents in natto on the type of raw soybean used.
Fig. 1. Linear fitting between the polyamine contents of raw soybeans and those of natto.
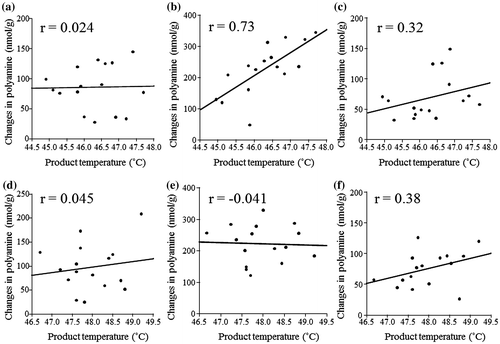
Yoshikawa et al.Citation19) reported that the sensory properties on natto were influenced by soybean cultivars and/or lines, indicating that raw soybean is strongly related to the process of bacterial fermentation. In addition, Kim et al.Citation20) reported that polyamine production of B. subtilis was correlated with microbial activity. Therefore, we also analyzed the correlations between product temperature, as an indicator of microbial activity, and each polyamine variations during fermentation. Under 39 °C fermentation, the Spd content showed a high positive relationship with product temperature (p = 0.00141, Fig. b), while no such correlation was observed for the other polyamines (Fig. a and c). In addition, there was no relationship in polyamine contents with temperature under fermentation at 42 °C (Fig. d–f). These facts indicated that the reductions in Put and Spm during fermentation were independent of the fermentation conditions. However, the increases in Spd were associated with fermentation condition at 39 °C. Given the weak relationships under 42 °C fermentation, the bacterial Spd production could reach a maximum at high temperature.
Fig. 2. Linear fitting between maximum product temperature and changes in polyamine during fermentation.
These results demonstrate that the polyamine contents of natto are strongly dependent on those of raw soybeans, whereas the effects of fermentation by B. subtilis (natto) are relatively weaker. In other words, a high polyamine-containing soybean would be useful for enhancement of the polyamine contents of natto.
Comparison of polyamine contents among soybean cultivars
Previous studies indicated that polyamine contents of soybean were different between cultivars;Citation14,21) we collected 9 Japanese soybean cultivars in order to select the highest polyamine containing cultivar. Each cultivar had a distinctive polyamine composition as shown in Table . For example, ‘Nakasen-nari’ displayed the highest polyamine contents of all cultivars, but with a relatively wide dispersion. ‘Yukishizuka’ had the lowest Spm content, although the Put and Spd contents were moderate. Moreover, ‘Satonohohoemi’ displayed the lowest Put and Spd contents, although the Spm content was moderate.
Table 3. Comparison of polyamine contents among soybean cultivars.
According to the variety database,Citation22) ‘Natto-shoryu’, ‘Suzumaru’, ‘Kosuzu’, and ‘Suzuhonoka’ are closely related (Supplemental Fig. 1). These four cultivars had similar polyamine contents and rates in each polyamine, and in particular, their Spm rates were higher than other cultivars except for ‘Satonohohoemi’ (Table ). This result suggested that genotype could be related to polyamine level of soybean.
On the other hand, environmental factors might affect polyamine contents of soybean since each soybean was harvested in different locations. In addition, Glória et al.Citation13) reported that polyamine contents varied according to harvest year. However, in the present study, only ‘Suzumaru’ displayed lower Spd content in 2014 compared to that harvested in 2013 (Table vs. Table ), suggesting that the effects of harvest year on polyamine contents might be weaker than differences between cultivars.
Taken together, we selected ‘Nakasen-nari’ as a high polyamine-containing cultivar among 9 Japanese cultivars. However, the genetic and environmental effects on polyamine contents in soybean require further investigation.
Conclusions
This study clarified the relationships of polyamine contents between soybean cultivars and natto. The polyamine contents of natto showed strong dependencies on those of raw soybean, indicating that the use of a high polyamine-containing soybean would be effective for enhancing the polyamine contents of natto. Moreover, each soybean cultivar displayed a specific polyamine composition, and ‘Nakasen-nari’ was found to contain the highest levels of polyamines among all Japanese cultivars tested. Further studies are required to investigate the optimal cultivation methods and conditions (weather, temperature, and harvest location) for enhancement of polyamine contents. In addition, it is also important to research the relationship between genotype and polyamine contents.
Author contributions
Kazuya Kobayashi, Yuji Kubo, Kumiko Koguchi, Yoshihiro Hoshi, Ken-ichi Matsumoto, and Kuniyasu Soda conceived and designed the work. Kazuya Kobayashi, Yuichiro Horii, and Satoshi Watanabe performed the experiments and analyzed the data. Kazuya Kobayashi wrote the manuscript.
Disclosure statements
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Ministry of Agriculture, Forestry and Fisheries of Japan under Grant the Science and technology research promotion program for agriculture, forestry, fisheries and food industry [grant number 26055A].
Supplemental material
Supplemental material for this article can be accessed at http://dx.doi.org/10.1080/09168451.2016.1270738.
TBBB_1270738_Supplemental_Material.tif
Download TIFF Image (31.2 KB)Acknowledgments
We thank Dr Kazuei Igarashi, Dr Kazuki Kanazawa, and the Japan Natto Cooperative Society Federation for their kind advice during this study.
Notes
Abbreviations: Put, putrescine; Spd, spermidine; Spm, spermine.
References
- Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14.10.1042/bj20031327
- Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894.10.1002/iub.v61:9
- Kusano T, Berberich T, Tateda C, et al. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–381.10.1007/s00425-008-0772-7
- Igarashi K, Kashiwagi K. Polyamine transport in bacteria and yeast. Biochem J. 1999;344:633–642.10.1042/bj3440633
- Soda K, Kano Y, Chiba F, et al. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE. 2013;8:e64357.10.1371/journal.pone.0064357
- Okamoto A, Sugi E, Koizumi Y, et al. Polyamine content of ordinary foodstuffs and various fermented foods. Biosci Biotechnol Biochem. 1997;61:1582–1584.10.1271/bbb.61.1582
- Nishimura K, Shiina R, Kashiwagi K, et al. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006;139:81–90.10.1093/jb/mvj003
- Nishibori N, Fujihara S, Akatuki T. Amounts of polyamines in foods in Japan and intake by Japanese. Food Chem. 2007;100:491–497.10.1016/j.foodchem.2005.09.070
- Kalač P. Health effects and occurrence of dietary polyamines: a review for the period 2005-mid 2013. Food Chem. 2014;161:27–39.
- Kubo Y, Rooney AP, Tsukakoshi Y, et al. Phylogenetic analysis of Bacillus subtilis strains applicable to natto (fermented soybean) production. Appl Environ Microbiol. 2011;77:6463–6469.10.1128/AEM.00448-11
- Kobayashi K, Shimojo S, Watanabe S. Contribution of a fermentation process using Bacillus subtilis (natto) to high polyamine contents of natto, a traditional Japanese fermented soy food. Food Sci Technol Res. 2016;22:153–157.10.3136/fstr.22.153
- Toro-Funes N, Bosch-Fuste J, Latorre-Moratalla ML, et al. Biologically active amines in fermented and non-fermented commercial soybean products from the Spanish market. Food Chem. 2015;173:1119–1124.10.1016/j.foodchem.2014.10.118
- Soda K, Kano Y, Sakuragi M, et al. Long-term oral polyamine intake increases blood polyamine concentrations. J Nutr Sci Vitaminol. 2009;55:361–366.10.3177/jnsv.55.361
- Glória MBA, Tavares-Neto J, Labanca RA. Influence of cultivar and germination on bioactive amines in soybeans (Glycine max L. Merril). J Agric Food Chem. 2005;53:7480–7485.10.1021/jf0509310
- Liu JH, Wang W, Gong X, et al. Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci. 2015;6:827.
- Veciana-Nogués MT, Mariné-Font A, Vidal-Carou MC. Biogenic amines in fresh and canned tuna. Effects of canning on biogenic amine contents. J Agric Food Chem. 1997;45:4324–4328.10.1021/jf970092k
- Woolridge DP, Martinez JD, Stringer DE, et al. Characterization of novel spermidine/spermine acetyltransferase, BltD, from Bacillus subtilis. Biochem J. 1999;340:753–758.10.1042/bj3400753
- Forouhar F, Lee IS, Vujcic J, et al. Structural and functional evidence for Bacillus subtilis PaiA as a novel N1-spermidine/spermine acetyltransferase. J Biol Chem. 2005;280:40328–40336.10.1074/jbc.M505332200
- Yoshikawa Y, Chen P, Zhang B, et al. Evaluation of seed chemical quality traits and sensory properties of natto soybean. Food Chem. 2014;153:186–192.10.1016/j.foodchem.2013.12.027
- Kim B, Byun BY, Mah JH. Biogenic amine formation and bacterial contribution in Natto products. Food Chem. 2012;135:2005–2011.10.1016/j.foodchem.2012.06.091
- Righetti L, Tassoni A, Bagni N. Polyamines content in plant derived food: a comparison between soybean and Jerusalem artichoke. Food Chem. 2008;111:852–856.10.1016/j.foodchem.2008.04.061
- AGROPEDIA [Internet]. Tsukuba, Japan: Tsukuba Business-Academia Cooperation Support Center, Agriculture, Forestry and Fisheries Research Council Secretariat; [cited 2016 Oct 6]. Available from: http://www.agropedia.affrc.go.jp/agriknowledge/hinshu
