Abstract
Encapsulating fish oil by spray drying with an adequate wall material was investigated to determine if stable powders containing emulsified fish-oil-droplets can be formed. In particular, the dextrose equivalent (DE) of maltodextrin (MD) affects the powder structure, surface-oil ratio, and oxidative stability of fish oil. The carrier solution was prepared using MD with different DEs (DE = 11, 19, and 25) and sodium caseinate as the wall material and the emulsifier, respectively. The percentage of microcapsules having a vacuole was 73, 39, and 38% for MD with DE = 11, 19, and 25, respectively. Peroxide values (PVs) were measured for the microcapsules incubated at 60 °C. The microcapsules prepared with MD of DE = 25 and 19 had lower PVs than those prepared with MD of DE = 11. The difference in PV can be ascribed to the difference in the surface-oil ratio of the spray-dried microcapsules.
Graphical abstract
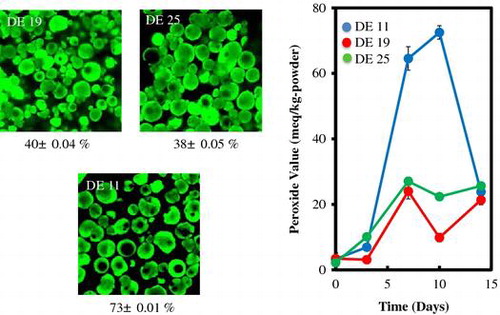
CLSM images for MDs of three DE and vacuole percentage were shown. PV changes of these powders at 60 °C were affected with these powder vacuole structures.
Fish oil is a source of n-3-polyunsaturated fatty acids with high amounts of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) , which are recommended for dietary consumption because of several beneficial effects: reduction of plasma triacylglycerol concentration, an anti-inflammatory effect, and a protective effect against coronary heart disease.Citation1) Unsaturated n-3 fatty acids are susceptible to oxidation, resulting in the formation of toxic hydroperoxides and off-flavors, and faster biodegradation. To prevent the oxidation of these fatty acids and to avoid the formation of off-flavors and toxic compounds, microencapsulation is used in the food industry to protect marine-based fish oil. Spray drying is the most common technique used to encapsulate food ingredients because it is inexpensive and the equipment is readily available.Citation2) Spray drying is the process by which an emulsion is atomized in a hot gas current to obtain powder. The process is divided into two main steps: the formation of emulsion with homogenization to form small oil-droplets in a solution, and spray drying with atomization and dehydration to form particles. Preparing the emulsion consists of adding a core material (such as fish oil) to a wall material (carbohydrates are the most commonly used). The emulsion is then homogenized: the core and the wall materials are mixed together. This operation affects the oil-droplet diameter and the distribution in spray-dried powder.
VehringCitation3) reviewed developments in particle engineering via spray drying. He found the morphology of spray-dried powder was affected by process parameters including wall materials. Tonon et al.Citation4) investigated the influence of process conditions on the physico-chemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. With respect to morphology, particles produced at a higher temperature were larger and many had a smooth surface. Shen et al.Citation5) investigated the oxidative stability of microencapsulated fish oil powders stabilized with blends of chitosan, modified starch, and glucose. Drush et al.Citation6) investigated the oxidative stability of fish oil microencapsulated by spray drying using different types of a matrix of n-octenylsuccinate-derivatized starch and either glucose syrup or trehalose. Legako and DunfordCitation7) investigated the encapsulation of fish oil in whey protein isolated by spray drying. Aghbashlo et al.Citation8) investigated the integrated optimization of the fish oil microencapsulation process by spray drying using three mathematical approaches and measured the fitness value of a genetic algorithm. There have been several studies on encapsulation of fish oil using various wall materials (carrier matrix) by spray drying as described above. However, no guidelines for selecting wall material have been established to obtain stable fish oil encapsulated in wall material.
There are several papers on the effect of preparation methods by spray drying and with MD of different dextrose equivalents on the physical properties and oxidation stability as follows. For the wall material for black mulberry juice, Fazaeli et al.Citation9) concluded that gum arabic and MD (DE = 6) were better for physical aspect analysis. Soottitantawat et al.Citation10) reviewed the encapsulation of hydrophilic compounds using different MDs and indicated that lower DE values resulted in higher retention of compounds during spray drying. However, oxidation stability analysis for the encapsulation of orange oil by Anandaraman et al.Citation11) showed an increasing DE value in MD could maintain the orange oil in the powder. Kagami et al.Citation12) reported that MD with a higher DE value was better for encapsulated fish oil spray-dried powder. Hogan et al.Citation13) also claimed that a larger value of DE at 28 was better for minimizing the destabilization of oil-droplets during spray drying and maximizing effectiveness in encapsulation.
In this study, the effect of dextrose equivalents 11, 19, and 25 of MD on the stability of fish oil in spray-dried powder was investigated by measuring the peroxide value (PV) of surface fish oil, encapsulated fish oil, and total fish oil in spray-dried powder stored at 60 °C. The physical properties of the spray-dried powder were also investigated.
Materials and methods
Materials
Sodium caseinate (protein 94%) was obtained from Mitsubishi Kagaku Foods Co., Ltd. (Tokyo, Japan). Maltodextrin (MD, DE = 11, 19, 25) was supplied by Matsutani Chemical Industry Co., Ltd. (Itami, Japan). Fish oil was purchased from NOF Corporation (Yokohama, Japan). All reagents used were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Preparation of feed emulsions
A wall material solution was prepared by dissolving MD in distilled water at 80 °C and cooling to room temperature. The fish oil was blended with the solution to give 60 wt% solid content. The composition of the feed solution was 24 wt% fish oil, 1.8 wt% casein, 34.2 wt% MD, and 40 wt% distilled water. The solution was mixed using a rotor-stator type homogenizer (Polytron, PT-6100; Kinematica, Littau, Switzerland) at 8,000 rpm for 3 min with a 30 s interval after 1 min and 2 min. The matrix solution was further homogenized using a high-pressure homogenizer (LAB2000; SMT Co., Ltd., Tokyo, Japan) at 25 MPa for 4 min.
Viscosity measurements
Viscosity of feed emulsion was measured at 50 °C using an R/S Plus Rheometer model DV-II (Brookfield Engineering Laboratories, Inc., MA, USA).
Spray drying
The feed emulsions were spray-dried using a pilot scale spray dryer (Ohkawara-L8; Ohkawara Kakouki Co., Ltd., Yokohama, Japan) equipped with a centrifugal atomizer. The detailed configuration of the spray dryer has been described elsewhere.Citation14) The feed rate was 25 mL/min, the atomizer speed was 30,000 rpm, and the air flow rate was set at 110 kg/h. In the drying process, the temperatures of inlet and outlet air were 140 °C and 70 to 95 °C. After being cooled to room temperature, the collected powders were stored in closed glass containers at −80 °C until use.
Analysis of oil-droplet and powder-particle diameters
The size distributions of the oil-droplets in the feed and reconstituted emulsions and the spray-dried powder particles were measured with a laser diffraction particle size analyzer (SALD-7100; Shimadzu Corporation, Kyoto, Japan) equipped with a batch sample cell. The reconstituted emulsions were obtained by dissolving the spray-dried powders in distilled water. The feed and reconstituted emulsions were pipetted directly into the cell containing distilled water for respective measurements. Meanwhile, the particle size distributions of the spray-dried powders were analyzed by dispersing the powder in 2-methyl-1-propanol. The volume-based diameter (D43) was considered to be the mean diameter for all measurements.
Measurement of surface–oil and total oil contents of the spray-dried powders
The spray-dried fish oil powder (0.3 g) was dispersed in 5 mL of hexane and vortexed for 15 min (Vortex Gene2; Scientific Ind. Inc., New York, USA). Total oil was analyzed by dissolving 20 mg of powder in 1 mL of dimethylformamide (DMF) in a glass bottle as described by Shiga et al.Citation15). Hexane (1 mL) was then added and the glass bottle was vortexed using the vortex mixer. The post-wash hexane (1 μL) was spotted onto a rod (S-III chromarod, Iatroscan; Mitsubishi Chemical Medience Corporation, Tokyo, Japan). The oil content was quantified for the rods using an Iatroscan MK-5 TLC-FID (Iatron Laboratories Inc., Tokyo, Japan).
The surface-oil ratio was defined by Equation (Equation1(1) ).
(1)
The oil encapsulation efficiency was calculated using Equation (Equation2(2) ).
(2)
Scanning electronic microscopy
Surface and cross-cut images of encapsulated fish oil powders were observed by a JSM 6060 Scanning electronic microscopy (SEM) (JEOL, Tokyo, Japan). The samples were placed onto the SEM sample holder using double-sided tape and coated with Pt-Pd using an MSP-IS Magnetron Sputter (Vacuum Device, Inc., Tokyo, Japan). The cross-cut structures were prepared using procedure as described by Soottitantawat et al.Citation16)
Confocal laser scanning microscope
A Confocal laser scanning microscope (CLSM) was used to measure vacuole diameter and number in the spray-dried powder with 40 mg of sodium fluorescein (1 mg/mL in 20 mmol/L NaHCO3/NaOH, pH 8.9) added to 100 g of feed solution. Green fluorescence images were measured using a CLSM FV1000-D BX61 (Olympus Corporation, Tokyo, Japan) with an excitation wavelength of 488 nm, which allowed emission of sodium fluorescein with an excitation/emission wavelength of 485/530 nm. The vacuole percentage of the spray-dried powder was defined as the ratio of the number of particles with a vacuole to the total number of counted particles.
PV of fish oil
The spray-dried powders (5 g) were placed in test tubes (φ24 × 90 mm) held at 60 °C with a heat block (DTU-1B; Taitec Corporation, Saitama, Japan). After incubating the samples, the PV was determined using the DMF method based on the AOCS Cd 8–53 acetic acid-chloroform procedure 21.Citation17) The sample (1 g) was dissolved in DMF (9 mL) under sonication, followed by mixing with a chloroform–acetic acid mixture (3:2, v/v; 80 mL); a saturated solution of potassium iodide (1 mL) was then added. The mixture was stirred for 1 min and then kept in the dark for 5 min. After adding distilled water (80 mL), the mixture was titrated against sodium thiosulfate (2 mmol/L) using a potentiometric titration system (East Ox Titrator; Mettler-Toledo International Inc., Switzerland). A blank mixture was also analyzed under similar conditions. The PV (meq / kg oil) was computed using Equation (Equation3(3) ).
(3)
where C is the concentration of sodium thiosulfate (mol/L); VS and VB are the volumes of sodium thiosulfate exhausted by the sample and the blank, respectively (L); and M is the total oil content in the spray-dried powder (kg). In the basis of powder weight of PV, M changed to the weight of powder.
Results and discussion
Reconstituted oil-droplet and powder-particle diameters
Figure shows the volume-based particle size distributions of the oil-droplets in feed emulsions and spray-dried powders. The diameter distribution of the reconstituted oil-droplets for DE = 11 was a little narrower than those for DE = 19 and 25. However, the average diameter of the reconstituted oil-droplets for DE = 11, 19, and 25 was about 1 μm. On the other hand, the powder-particle diameter showed almost similar volume distribution. Despite that, the average diameter of the powder particles was 48 μm for DE = 11, 38 μm for DE = 19, and 41 μm for DE = 25 of MD as shown in Table .
Fig. 1. Volume-based particle diameter distributions of reconstituted oil-droplet and particle diameter in spray-dried powder.
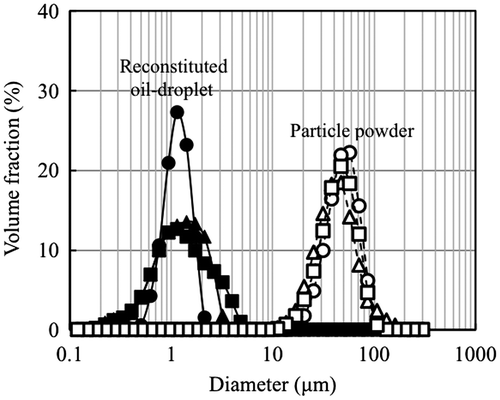
Table 1. Physical properties of the spray-dried powder
SEM and CLSM images
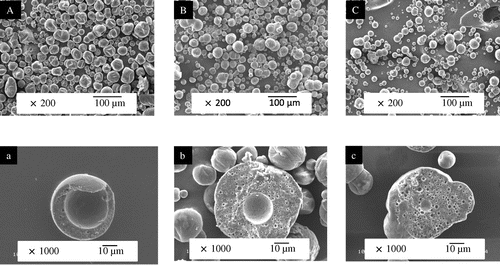
The morphology of the surface and cross-cut structures of the spray-dried powder was observed using SEM and CLSM. Figure shows SEM images of the surface and cross-cut structures for the spray-dried powder for three different DEs (11, 19, and 25) of MD. The spray-dried powder had some wrinkles and many dents on the surface of particles for DE = 11. Vacuoles could be found in the cross-cut images of those powders and the vacuole size for DE = 11 was larger than other MD of DE = 19 and 25. Gharsallaoui et al.Citation18) showed the microencapsulation of lipophilic ingredients with pea protein isolates as emulsifier using MD of DE = 6, 12, 19, and 28 as wall material. They observed particles with higher DE were spherical and had smooth surface spray-dried powder, whereas the powder with lower DE was disrupted and the particles had many dents. Furthermore, they suggested that MD with high DE might act as a plasticizer and prevent irregular shrinkage. Sarkar et al.Citation19) showed the stability of spray-dried emulsion with ultra-high oil content and defined the dents in the powder as observed on SEM images as ‘free-oil droplets.’ They considered that the free-oil-droplets could be extracted with hexane during extraction analysis. Soottitantawat et al.Citation10) also reviewed surface shrinkage structures powder were affected by drying rate conditions and DE of MD values.
Fig. 2. SEM images of surface (A, B, C) and cross-cut structures (a, b, c) of spray-dried powder. MD (DE = 11): A, a; MD (DE = 19): B, b; and MD (DE = 25): C, c.
Spray-dried powder particles with a vacuole could be observed by the green fluorescence ring, and the diameter of each particle and vacuole was measured. Figure shows CLSM images representing the internal structure of the spray-dried powder with MD of DE = 11, 19, and 25. In counting 400 particles, the vacuole percentages of the spray-dried powder were 73 ± 0.01% for MD with DE = 11, 40 ± 0.04% for DE = 19, and 38 ± 0.05% for DE = 25. The vacuole diameter was also measured for 30 particles of the cutting images: 24 ± 12 μm for MD with DE = 11, 8.6 ± 5.6 μm for DE = 19, and 5.8 ± 3.6 μm for DE = 25. These vacuole percentages showed that powder prepared with MD and DE = 11 had not only a higher number of vacuoles but also larger vacuole diameters. MD with low DE produces high viscosity emulsions.Citation13,20) In our studies, the viscosities of feed emulsion for MD with DE = 11 were 231 mPa s, when the DE decreased, the viscosities decreased to 48 mPa s for DE = 19 and 44 mPa s for DE = 25. Paramita et al.Citation21) reported on the effect of additives on the morphology of spray-dried powder, finding the vacuole percentage in spray-dried powder is not affected by viscosity but is influenced by outlet air temperature during spray drying and the wall material composition.
Physical properties of the spray-dried powder
The physical properties of the spray-dried powder are summarized in Table . The oil encapsulation efficiency increased from 74% to about 90% on increasing DE values from 11 to 19 and 25. The moisture content of the fish oil powder prepared with MD of DE = 11, 19, and 25 was about 2.8–2.5%. The surface-oil ratio for MD of DE = 11 was 26% and almost two times that for DE = 19 and 25. This result suggested that larger vacuole diameter and dents on the powder surface might affect the higher surface-oil ratio for powder prepared with MD of DE = 11 as shown in Table . The vacuole size and number in the spray-dried powder affected the surface-oil ratio in the spray-dried powder. Kikuchi et al.Citation22) investigated the effects entire of oil content and oil-droplet size on the surface-oil ratio using percolation theory. They showed that the surface-oil ratio was lower at lower oil contents, and the existence of vacuoles markedly influenced surface-oil ratio. The percolation model suggested the surface oil lower at smaller vacuole-diameter inside the particle powder. This result indicated larger vacuole of particle powder enhanced the oil content at the surface layer of particle powder.
Stability of fish oil in the spray-dried powder
The stability of fish oil in the spray-dried powder was measured at a storage temperature of 60 °C. Surface oil was washed with hexane and concentrated using a rotary evaporator. Encapsulated oil was measured using the washed powder. The PV of total oil was measured using the DMF method with iodometric titration. Using the solubilization of the incubated spray-dried powder by DMF, the PV of the powders was easily measured without having to extract the fish oil from the powder. Figure shows the PV changes in the spray-dried powders based on the weight of the oil for surface oil (A), encapsulated oil (B), and total oil (C), and based on the weight of the powder for surface oil (a), encapsulated oil (b), and total oil (c) stored at 60 °C. PV in basis of weight of powder was calculated using the value of M divided by the total oil content in the powder.
Fig. 4. PVs of spray-dried powders stored at 60 °C for (I) based on the weight of oil and (II) based on the weight of powder.
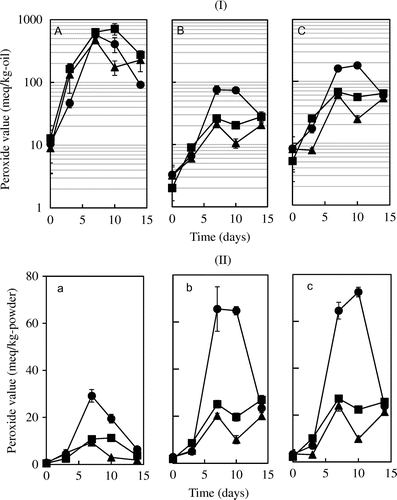
As shown in Figure (A), surface oils oxidized significantly faster than encapsulated oils for three MDs (DE = 11, 19, and 25). Ahn et al.Citation23) also found higher PVs of surface oil in encapsulated seed oil kept at 60 °C for 30 days. They concluded that optimizing the microencapsulation condition could reduce surface-oil oxidation. Surface-oil oxidation was not affected by the DE of MD and was much the same during the storage time. On the other hand, the DE of MD affected the PVs of the encapsulated oils. Encapsulated oil for MD of DE = 11 had higher oxidation than those for MD of DE = 19 and 25. This PV behavior could be seen in the plot of PV changes based on powder weight as shown in Fig. (b) and (c). Wang and ZhouCitation24) in their study of encapsulated soy sauce demonstrated that higher DE values of MD had higher stability in terms of caking strength. These data indicated that the surface-oil ratio and selection of wall material is important in forming stable fish-oil powder by spray drying, as well as for other encapsulated oil powders.
Soottitantawat et al.Citation25) studies the effect of water activity on the spray-dried d-lemonene powder with gum Arabic, soybean water-soluble polysaccharide or modified starch blended with MD (DE = 20) stated the stability of flavors that can be relate to oxidation that lean on the glass transition (Tg) wall materials. Generally, the Tg temperature of MD increases when the DE value decreases because of the degree of polymerization. A review by Bhandari et al.Citation26) on implication of Tg for the drying and stability of dried foods disclosed the Tg temperature of MD (DE = 36, 25, 10 and 5) was increasing from 100 to 188 °C.
Several researchers investigated the stability of functional lipids and flavors in spray-dried powder.Citation27–30) However, the focus of these studies was not on the effects of wall material such as in different DE value of MD and oil-droplet size in powders on the oxidation stability. Encapsulation of stable oil might consider the mass transfer of oxygen in the matrix of spray-dried powder.Citation31)
In our experiment, powder was prepared with almost same diameter of oil-droplet and particle of powders. These parameters are crucial to control since the surface-oil content affects the initial oxidation stability.
Conclusion
MD of DE = 11 produced microcapsules having larger and more vacuoles. The percentage of microcapsules having a vacuole was 73, 39, and 38% for MD of DE = 11, 19, and 25, respectively. PVs were measured for those microcapsules incubated at 60 °C; microcapsules prepared with MD of DE = 25 and 19 had lower PVs than those prepared with MD of DE = 11. The different results for PV can be ascribed to the difference in the surface–oil content of the spray-dried microcapsules.
Author contributions
H.S., T.L.N. and H.Y. conceived and designed the study. S.A. and H.S. carried out most of the experiments. A.A.G., S.A. and H.S. analyzed the data. A.A.G. wrote the manuscript. S.A. and H.Y. reviewed and edited the manuscript.
Funding
A.A.G. received a scholarship from the Malaysian Ministry of Education throughout the study period. This work was supported by a Scientific Research Grant from the Mishima Kaiun Memorial Foundation and the Japan Society for Promotion of Science JSPS KAKENHI [grant number 15K07455].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Heinzelmann K, Franke K. Using freezing and drying techniques of emulsions for the microencapsulation of fish oil to improve oxidation stability. Colloids Surf B. 1999;12:223–229.10.1016/S0927-7765(98)00077-0
- Gharsallaoui A, Roudaut G, Chambin O, et al. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int. 2007;40:1107–1121.10.1016/j.foodres.2007.07.004
- Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25:999–1022.10.1007/s11095-007-9475-1
- Tonon RV, Brabet C, Hubinger M. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J Food Eng. 2008;88:411–418.10.1016/j.jfoodeng.2008.02.029
- Shen Z, Augustin MA, Sanguansri L, et al. Oxidative stability of microencapsulated fish oil powders stabilized by blends of chitosan, modified starch, and glucose. J Agric Food Chem. 2010;58:4487–4493.10.1021/jf904102k
- Drusch S, Schwarz K. Microencapsulation properties of two different types of n-octenylsuccinate-derivatised starch. Eur Food Res Technol. 2006;222:155–164.10.1007/s00217-005-0020-3
- Legako J, Dunford NT. Effect of spray nozzle design on fish oil-whey protein microcapsule properties. J Food Sci. 2010;75:E394–E400.10.1111/j.1750-3841.2010.01708.x
- Aghbashlo M, Mobli H, Madadlou A, et al. Integrated optimization of fish oil microencapsulation process by spray drying. J. Microencapsulation. 2012;29:790–804.10.3109/02652048.2012.692398
- Fazaeli M, Emam-Djomeh Z, Kalbasi Ashtari A, et al. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod Process. 2012;90:667–675.10.1016/j.fbp.2012.04.006
- Soottitantawat A, Partanen R, Neoh TL, et al. Encapsulation of hydrophilic and hydrophobic flavors by spray drying. Jpn J Food Eng. 2015;16:37–52.
- Anandaraman S, Reineccius GA. Stability of encapsulated orange peel oil. Food Technol. 1986;40:88–93.
- Kagami Y, Sugimura S, Fujishima N, et al. Oxidative stability, structure, and physical characteristics of microcapsules formed by spray drying of fish oil with protein and dextrin wall materials. J Food Eng. 2003;68:2248–2255.
- Hogan SA, McNamee BF, O’Riordan ED, et al. Emulsification and microencapsulation properties of sodium caseinate/carbohydrate blend. Int Dairy J. 2001;11:137–144.10.1016/S0958-6946(01)00091-7
- Paramita V, Iida K, Yoshii H, et al. Effect of feed liquid temperature on the structural morphologies of d-limonene microencapsulated powder and its preservation. J Food Sci. 2010;75:E39–E45.10.1111/jfds.2010.75.issue-1
- Shiga H, Adachi S, Adachi S, et al. A simple method for determining the flaxseed or fish oil content with N, N dimethylformamide in microcapsules prepared by spray drying. Jpn J Food Eng. 2014;15:131–139.
- Soottitantawat A, Yoshii H, Furuta T, et al. Microencapsulation by spray drying: influence of emulsion size on the retention of volatile compounds. J Food Sci. 2003;68:2256–2262.10.1111/jfds.2003.68.issue-7
- Official methods and recommended practices of the American Oil Chemists’ Society. Cd 8-53. Peroxide value acetic acid-chloroform. 4th ed. Champaign (IL): AOCS Press; 1997.
- Gharsallaoui A, Saurel R, Chambin O, et al. Pea (Pisum sativum, L.) protein isolate stabilized emulsions: a novel system for microencapsulation of lipophilic ingredients by spray drying. Food Bioprocess Technol. 2012;5:2211–2221.
- Sarkar A, Arfsten J, Golay PA, et al. Microstructure and long-term stability of spray dried emulsions with ultra-high oil content. Food Hydrocolloid. 2016;52:857–867.10.1016/j.foodhyd.2015.09.003
- Di Mattia C, Paradiso VM, Andrich L, et al. Effect of olive oil phenolic compounds and maltodextrins on the physical properties and oxidative stability of olive oil o/w emulsions. Food Biophys. 2015;9:396–405.
- Paramita V, Iida K, Yoshii H, et al. Effect of additives on the morphology of spray-dried powder. Drying Technol. 2010;16:3233–3233.
- Kikuchi K, Yamamoto S, Shiga H, et al. Surface oil content of microcapsules containing various oil fractions and oil-droplet sizes. Jpn J Food Eng. 2013;14:169–173.
- Ahn JH, Kim YP, Lee YM, et al. Optimization of microencapsulation of seed oil by response surface methodology. Food Chem. 2008;107:98–105.10.1016/j.foodchem.2007.07.067
- Wang W, Zhou W. Characterization of spray-dried soy sauce powders using maltodextrins as carrier. J Food Eng. 2012;109:399–405.10.1016/j.jfoodeng.2011.11.012
- Soottitantawat A, Yoshii H, Furuta T, et al. Effect of water activity on the release characteristics and oxidative stability of d-lemonene encapsulated by spray drying. J Agric Food Chem. 2004;52:1269–1276.10.1021/jf035226a
- Bhandari BR, Howes T. Implication of glass transition for the drying and stability of dried foods. J Food Eng. 1999;40:71–79.10.1016/S0260-8774(99)00039-4
- Drusch S, Serfert Y, Scampicchio M, et al. Impact of physicochemical characteristics on the oxidative stability of fish oil microencapsulated by spray-drying. J Agric Food Chem. 2007;55:11044–11051.10.1021/jf072536a
- Shen Z, Augustin MA, Sanguansri L, et al. Oxidative stability of microencapsulated fish oil powders stabilized by blends of chitosan, modified starch, and glucose. J Agric Food Chem. 2010;58:4487–4493.10.1021/jf904102k
- Domian E, Sulek A, Cenkier J, et al. Influence of agglomeration on physical characteristics and oxidative stability of spray-dried oil powder with milk protein and trehalose wall material. J Food Eng. 2014;125:34–43.10.1016/j.jfoodeng.2013.10.017
- Cano-Higuita DM, Malacrida CR, Telis VRN. Stability of curcumin microencapsulated by spray and freeze drying in binary and ternary matrices of maltodextrin, gum arabic and modified starch. J Food Process Preserv. 2015;39:2049–2060.10.1111/jfpp.12448
- Abd Ghani A, Matsumura K, Yamauchi A, et al. Effects of oil-droplet diameter on the stability of squalene oil in spray-dried powder. Drying Technol. 2016;34:1726–1734.10.1080/07373937.2016.1190936

