Abstract
We assessed the effect of sulforaphene (SFE) on osteoclast differentiation. SFE significantly decreased the number of RANKL-induced tartrate-resistant acid phosphatase-positive cells and suppressed pre-osteoclast multinucleation. Furthermore, SFE downregulated mRNA expression of DC-STAMP, OC-STAMP, and Atp6v0d2, which encode cell–cell fusion molecules. Our data suggest that SFE attenuates pre-osteoclast multinucleation via suppression of cell–cell fusion.
Bone quantity is controlled by the balance between bone resorption by osteoclasts and bone formation by osteoblasts.Citation1) Inhibition of osteoclast differentiation is effective in suppressing osteoporosis. Receptor activator of nuclear factor kappa-B ligand (RANKL) is an essential factor for osteoclast differentiation and is expressed in osteoblasts. RANKL specifically binds to its receptor, RANK, and activates the c-Fos pathway.Citation2) Additionally, this signal results in the activation of nuclear factor of activated T cells c1 (NFATc1), a master regulator of osteoclast differentiation, which controls the expression of the genes encoding tartrate-resistant acid phosphatase (TRAP) and cathepsin K (Ctsk).Citation3) Importantly, osteoclast cell fusion is essential for pre-osteoclast multinucleation. Osteoclast cell fusion through cell–cell fusion molecules, such as dendritic cell-specific transmembrane protein (DC-STAMP), osteoclast stimulatory transmembrane protein (OC-STAMP), and v-ATPase V0 subunit d2 (Atp6v0d2), is one of the main factors involved in the regulation of bone resorption.Citation4–6) Sulforaphene (SFE) (Fig. (A)), a sulfur-containing compound, is derived from Japanese radish, a kind of cruciferous vegetable, and induces apoptosis in several cancer cell lines.Citation7,8) Recently, we demonstrated that sulforaphane inhibits osteoclast differentiation by suppressing the cell–cell fusion molecules, DC-STAMP and OC-STAMP. Citation9) However, the molecular function of SFE, an isothiocyanate (ITC) with a double bond, in inhibiting osteoclast differentiation has not been documented. In the present study, we investigated the effects of SFE on pre-osteoclast multinucleation and cell fusion in vitro.
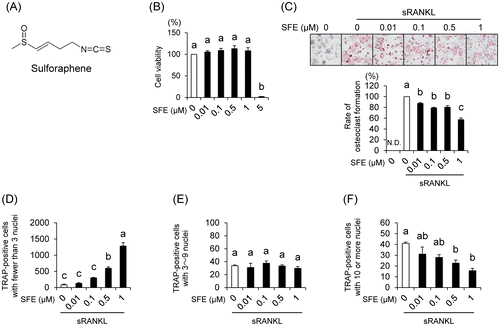
Fig. 1. Effects of sulforaphene (SFE) on osteoclast formation in RAW 264.7 cells.
SFE was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). RAW 264.7 murine macrophages/monocytes were purchased from American Type Culture Collection (Manassas, VA, USA). In the culture system, RAW 264.7 cells were cultured in α-minimal essential medium (α-MEM) (phenol red-free) (Gibco BRL/Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Biowest, Nuaillé, France), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco BRL/Invitrogen) at 37 °C under 5% CO2. Total RNA was isolated from RAW 264.7 cells by using the Sepasol-RNA I Super G (Nacalai Tesque, Tokyo, Japan). cDNA was synthesized from total RNA using the PrimeScript RT Reagent Kit (Takara Biotechnology, Otsu, Japan). Real-time PCR was performed using the ABI StepOnePlus System (Applied Biosystems, Foster City, CA, USA) with THUNDERBIRD qPCR Mix (Toyobo, Osaka, Japan). Sequences of primers used have been reported previously.Citation9) The following primer sets were used: Atp6v0d2, 5′-GACCCTGTGGCACTTTTTGTATTC-3′ (forward), and 5′-GCTTGCATTTGGGGAATCTATC-3′ (reverse). All reactions were normalized to the housekeeping gene β-actin. Results are presented as means ± S.E. Multiple comparisons were performed using Tukey’s test, after one-way analysis of variance (ANOVA). p < 0.05 was considered statistically significant.
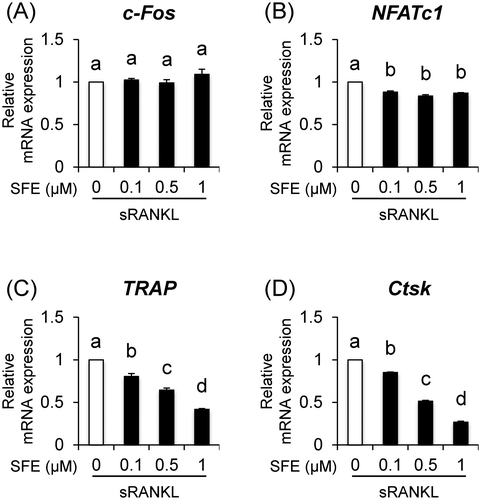
We examined the cytotoxic effect of SFE on RAW 264.7 cells, osteoclast precursor cells, using the Cell Counting Kit-8 (CCK-8; DOJINDO, Kumamoto, Japan) assay. Low-dose SFE had no cytotoxicity, but decreased cell viability at a concentration of 5 μM (Fig. (B)). These results indicate that the maximum concentration used in our subsequent experiments (1 μM) had no cytotoxic effects against RAW 264.7 cells. Next, to examine the effects of SFE on pre-osteoclast multinucleation, the cells were incubated with SFE in the presence of soluble RANKL (sRANKL; R&D Systems, Minneapolis, MN, USA). Compared to that reported for sRANKL-induced cells, SFE decreased the rate of multinucleated osteoclast formation (Fig. (C)). Interestingly, the osteoclasts with 10 nuclei or more decreased in number, whereas the cells with fewer than 3 nuclei increased drastically (Fig. (D–F)). Furthermore, we analyzed the effect of SFE on the mRNA expression levels of osteoclast-associated genes, such as c-Fos, NFATc1, TRAP, and Ctsk using real-time PCR. Compared to that in sRANKL-induced cells, SFE dose dependently decreased mRNA expression levels of TRAP and Ctsk, whereas the expression of c-Fos did not change (Fig. (A–D)). NFATc1 mRNA expression was downregulated by SFE at 0.l μM, but there was no dose dependency. Additionally, the effects of SFE on cell viability and pre-osteoclast multinucleation of bone marrow cells, which are closer to in vivo conditions, were similar to those observed with RAW 264.7 cells (Supplemental Fig. ; see Biosci. Biotechnol. Biochem Website). This result indicates that SFE inhibits osteoclast differentiation by suppressing RANKL/NFATc1 signal, but there might be other reasons.
Fig. 2. Effects of SFE on expression of osteoclast-differentiation associated genes.
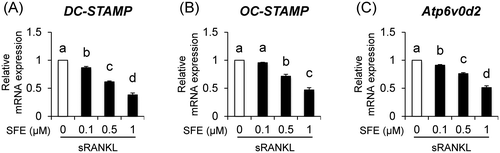
Monomer osteoclasts promote cell–cell fusion by increasing the expression levels of DC-STAMP or OC-STAMP, which are major factors for activating osteoclasts and inducing bone resorption.Citation10,11) Additionally, according to a previous study, Atp6v0d2-deficient mice exhibit impaired osteoclast cell fusion.Citation6) Thus, these reports indicate that DC-STAMP, OC-STAMP, and Atp6v0d2 play an important role in osteoclast cell fusion. We also found that SFE inhibited pre-osteoclast multinucleation and decreased the number of multinuclear cells (10 or more nuclei) as shown in Fig. (D–F). Therefore, we revealed that SFE affects osteoclast cell fusion. As shown in Fig. (A–C), SFE dose dependently decreased the mRNA expression levels of DC-STAMP, OC-STAMP, and Atp6v0d2 in sRANKL-induced multinucleated osteoclasts. Importantly, Kim et al. reported that NFATc1 induces the expression of DC-STAMP and Atp6v0d2 by binding to their promoter regions.Citation12) These data indicate that NFATc1/DC-STAMP and Atp6v0d2 signals play a key role in the pre-osteoclast multinucleation process. In addition, OC-STAMP is induced by sRANKL treatment, and it promotes the formation of multinucleated osteoclasts.Citation13)
Fig. 3. Effects of SFE on expression of osteoclast cell fusion molecules.
In conclusion, we demonstrated that SFE plays a novel role in inhibiting osteoclast cell fusion. In addition, SFE decreased the number of multinucleated osteoclasts by suppressing RANKL/NFATc1 signal. Particularly, this study was the first to demonstrate that SFE strongly inhibited multinucleation of mononuclear pre-osteoclasts by downregulating not only DC-STAMP and OC-STAMP, but also Atp6v0d2. Further studies of the mechanisms by which SFE improves bone resorption and osteoporosis in vivo are still required.
Author contributions
T. T., H. I, N. T., and M. U. designed the experiments. T. T. and H. I. performed the experiments. R. K-T. participated in cell culture. T. T., H. I., N. T., R. K-T., and M. U. analyzed the data. T. T., H. I., and M. U. wrote the manuscript. N. T. contributed to the development of the manuscript. All authors read and approved the final manuscript.
Supplemental materials
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2017.1281729.
Funding
This work was supported by Grant-in-Aid for Scientific Research (A) [No. 15H01767] from the Japan Society for the Promotion of Science (JSPS).
TBBB_1281729_Supplemental.zip
Download Zip (1.7 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
Abbreviations: SFE,sulforaphene; ITC, isothiocyanate; RANKL, Receptor activator of nuclear factor kappaB ligand; NFATc1, nuclear factor of activated T cells c1; TRAP, tartrate-resistant acid phosphatase; Ctsk, cathepsin K; DC-STAMP, dendritic-cell specific transmembrane protein; OC-STAMP, osteoclast stimulatory transmembrane protein; Atp6v0d2, v-ATPase V0 subunit d2; α-MEM, α-minimal essential medium; CCK-8,cell counting kit-8; BMCs, bone marrow cells.
References
- Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396.10.1196/annals.1365.035
- Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem Biophys Res Commun. 2003;305:211–214.10.1016/S0006-291X(03)00695-8
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342.10.1038/nature01658
- Zhang C, Dou CE, Xu J, et al. DC-STAMP, the Key fusion-mediating molecule in osteoclastogenesis. J Cell Physiol. 2014;229:1330–1335.10.1002/jcp.v229.10
- Khan UA, Hashimi SM, Bakr MM, et al. Foreign body giant cells and osteoclasts are TRAP positive, have podosome-belts and both require OC-STAMP for cell fusion. J Cell Biochem. 2013;114:1772–1778.10.1002/jcb.v114.8
- Lee SH, Rho J, Jeong D, et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12:1403–1409.
- Byun S, Shin SH, Park J, et al. Sulforaphene suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol Nutr Food Res. 2016;60:1068–1078.10.1002/mnfr.v60.5
- Yang M, Teng W, Qu Y, et al. Sulforaphene inhibits triple negative breast cancer through activating tumor suppressor Egr1. Breast Cancer Res Treat. 2016;158:277–286.10.1007/s10549-016-3888-7
- Takagi T, Inoue H, Takahashi N, et al. Sulforaphane inhibits osteoclast differentiation by suppressing the cell–cell fusion molecules DC-STAMP and OC-STAMP. Biochem Biophys Res Commun. 2017;483:718–724.
- Yagi M, Miyamoto T, Sawatani Y, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–351.10.1084/jem.20050645
- Miyamoto H, Suzuki T, Miyauchi Y, et al. Osteoclast stimulatory transmembrane protein and dendritic cell–specific transmembrane protein cooperatively modulate cell–cell fusion to form osteoclasts and foreign body giant cells. J Bone Miner Res. 2012;27:1289–1297.10.1002/jbmr.1575
- Kim K, Lee SH, Ha Kim J, et al. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol Endocrinol. 2008;22:176–185.10.1210/me.2007-0237
- Yang M, Birnbaum MJ, MacKay CA, et al. Osteoclast stimulatory transmembrane protein (OC-STAMP), a novel protein induced by RANKL that promotes osteoclast differentiation. J Cell Physiol. 2008;215:497–505.10.1002/(ISSN)1097-4652
