Abstract
Endo-1,3-β-glucanase from Cellulosimicrobium cellulans DK-1 has a carbohydrate-binding module (CBM-DK) at the C-terminal side of a catalytic domain. Out of the imperfect tandem α-, β-, and γ-repeats in CBM-DK, the α-repeat primarily contributes to β-glucan binding. This unique feature is derived from Trp273 in α-repeat, whose corresponding residues in β- and γ-repeats are Asp314 and Gly358, respectively. In this study, we generated Trp-switched mutants, W273A/D314W, D270A/W273A/D314W, W273A/G358W, and D270A/W273A/G358W, and analyzed their binding abilities toward laminarioligosaccharides and laminarin. While the binding affinities of D270A/W273A and W273A mutants were either lost or much lower than that of the wild-type, those of Trp-switched mutants recovered, indicating that a Trp introduction in β- or γ-repeat can substitute the α-repeat by primarily contributing to β-glucan binding. Thus, we have successfully engineered a CBM-DK that binds to laminarin by a mechanism different from that of the wild-type, but with similar affinity.
Non-catalytic carbohydrate-binding module (CBM) is often attached to catalytic unit of carbohydrate degrading enzymes, both of which fold independently. To date, CBMs have been classified into 80 different families based on the amino acid sequence, binding specificity, and three-dimensional structure.Citation1) Investigating the role of CBMs in enzymes has shown that they contribute to the catalytic activity and the stability of enzyme.Citation2,3) The carbohydrate binding analysis of CBMs can also help us understand the molecular recognition mechanism in carbohydrate–protein interactions, which can have wide range of applications in drug design and as diagnostic tools.Citation4,5)
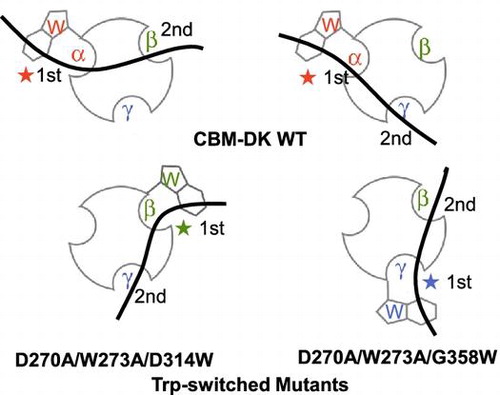
Endo-1,3-β-glucanase from Cellulosimicrobium cellulans DK-1 has a catalytic domain and a CBM (CBM-DK), classified in family 13, connected by a Gly/Ser-rich linker region.Citation6,7) The CBMs in family 13 have a β-trefoil fold displaying the pseudo-3-fold symmetry arising from imperfect tandem α-, β-, and γ-repeats, each of which has a putative carbohydrate-binding site.Citation8) We recently found that the α-repeat of CBM-DK primarily contributes to β-glucan binding,Citation9) a property different from other CBMs in family 13 from Streptomyces lividans xylanase 10A and Streptomyces olivaceoviridis E-86 xylanase.Citation10,11) The CBM13 lectin-like xylan binding domain from Streptomyces lividans xylanase 10A was reported to have different repeat preferences for the respective substrates.Citation12) Based on the available crystal structures, we had hypothesized that the conserved Asp residues, Asp270 in α-repeat, Asp311 in β-repeat, and Asp355 in γ-repeat would contribute to β-glucan binding. Using mutational experiments, we showed that Asp270 primarily contributes to the binding of laminarioligosaccharides and laminarin, and Asp311 and Asp355 additionally contribute to the multivalent binding of laminarin.Citation9) We also showed that the unique α-repeat preference for β-glucan binding of CBM-DK is due to the Trp273 residue in α-repeat, which could be exposed to the solvent and interact with β-glucan. In the amino acid sequence of CBM-DK, Trp273 corresponding residues in β- and γ-repeats are Asp314 and Gly358, respectively (Fig. ). The absence of Trp residues in β- and γ-repeats was hypothesized to result in the α-repeat contributing primarily to β-glucan binding. In this study, we substituted Trp at 273 with Ala, and switched the residues at 314 and 358 with Trp, as W273A/D314W and W273A/G358W mutations, respectively. The effects of these mutations on β-glucan binding were analyzed using surface plasmon resonance (SPR) biosensor and isothermal titration calorimetry (ITC). As β-glucans, both laminarioligosaccharides and laminarin were used. The binding of laminarioligosaccharides to CBM-DK is monovalent, while that of laminarin is multivalent, due to its longer chain. In the SPR system, the multivalent binding of laminarin can involve several CBM molecules immobilized on the sensor chip, increasing the binding strength depending on the laminarin concentration, as reported previously.Citation9) In contrast, ITC can detect the interaction of laminarin with a single CBM-DK molecule. The SPR and ITC results clearly showed that the α-repeat preference of CBM-DK for β-glucan binding could be switched by Trp introduction in other repeats.
Fig. 1. The amino acid sequence of CBM-DK. The sequence alignments of α-, β-, and γ-repeats were carried out based on the 3D structure model of CBM-DK, reported previouslyCitation9). Single dashes denote identity to α-repeat, and double dashes indicate no amino acid residue at that position.

Materials and methods
Materials
Laminarin from Laminaria digitata and laminarioligosaccharides from Poria cocos were purchased from Sigma–Aldrich Co. (USA) and Seikagaku Kogyo Co. (Japan), respectively. The plasmid vector, pET28a (Novagen, USA), was used for expression of CBM-DK and its mutant proteins.
The plasmid DNAs of CBM-DK mutant proteins were obtained by site-directed mutagenesis. The protein with an N-terminal polyhistidine-tag was overexpressed in E. coli, and purified as described previously.Citation9) The protein concentration was calculated from absorbance of 280 nm using molar extinction coefficient, 3.5 × 104 M−1cm−1 for the wild-type and its modified values for the mutants, depending on the number of Trp.
Circular dichroism (CD) measurements
Far-UV (200–250 nm) CD spectra were measured on a Jasco J-820 spectropolarimeter at 20 °C equipped with Peltier-type temperature control system. The spectra were obtained for the protein concentration, 0.02 mg/ml, in 40 mM potassium phosphate buffer (pH 7.0), using quartz cell with 1.0-cm path-length. CD spectra were obtained using scanning speed of 20 nm/min, a time response of 1 sec, a bandwidth of 1 nm, and an average over 4 scans. The melting curves were recorded in temperature mode at 230 nm, from 20 to 80 °C with a heating rate of 1.0 °C/min. The analysis of the transition curves obtained by temperature-scanning CD measurements was performed on the basis of two-state transition model, as described previously.Citation13)
Analytical ultracentrifugation (AUC) measurements
Analytical ultracentrifugation sedimentation velocity (AUC-SV) experiments were performed using a Beckman Optima XL-A analytical ultracentrifuge equipped with the 4-hole An60 Ti rotor at 20 °C, as described previously.Citation14) The concentration of CBM-DK was 37 μM in 40 mM potassium phosphate buffer (pH 7.0) and the speed of rotation was 60,000 rpm. A radial step size of 0.003 cm was used for scanning which was carried out for 2 min in continuous scan mode and data were collected using absorbance optics at 290 nm. The distribution of sedimentation coefficient was analyzed using c(s) method of Program SEDFIT.Citation15)
SPR measurements
SPR experiments were carried out at 25 °C using Biacore 2000 (GE Healthcare) as described previously.Citation9) CBM-DK proteins were immobilized on the dextran surface of a CM5 sensor chip (GE Healthcare) with amine coupling method. The β-glucan analyzed was diluted in PBS containing 0.005% Tween 20 (running buffer) and the association of each solution was recorded at rate of 20 μl/min for 3 min. The sensorgrams obtained were first examined by adjusting for background changes reflected in the bulk refractive index, using sensorgrams of interactions between β-glucan and negative control dextran surface on the chip. The equilibrium association constant (Ka) values were determined by Scatchard analysis.
ITC measurements
ITC experiments were carried out at 25 °C using iTC200 (Malvern) as described previously.Citation9) All samples were in 40 mM phosphate buffer (pH 7.0). The solution of laminarin was titrated into the solution of CBM-DK proteins. The heat for each injection was subtracted from the dilution heat of the titrant. Each corrected heat was divided by the moles of laminarin injected and was analyzed using the Origin software supplied by Malvern.
Results
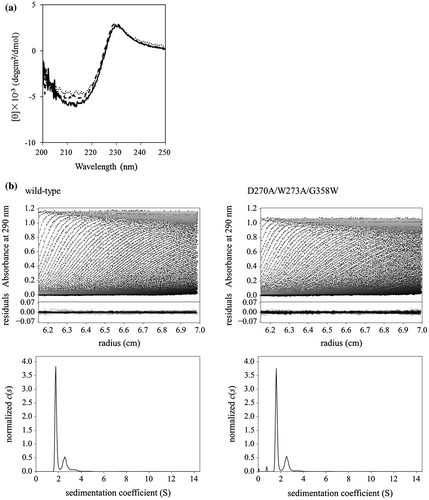
Based on the assumption that Trp273 of CBM-DK is the key residue responsible for the role of α-repeat in β-glucan binding, we modified Trp273 deleted mutants, W273A and D270A/W273A, by substituting the residues Asp314 and Gly358, corresponding to Trp273 in β- and γ-repeats, respectively, with Trp (Fig. ). As reported previously,Citation9) D270A mutant lost the laminarin binding ability, while W273S mutant showed reduced activity. The introduction of Trp in β- and γ-repeats in the mutants with decreased binding affinities was expected to detect whether Trp-focused mutations can restore β-glucan binding affinities. We overexpressed CBM-DK and its mutants, D270A/W273A, W273A, W273A/D314W, W273A/G358W, D270A/W273A/D314W, and D270A/W273A/G358W, with an N-terminal His-tag. Purification yielded proteins with more than 95% purity, as estimated by SDS-PAGE analysis (data not shown). The far-UV CD spectra of purified CBM-DK and its mutant proteins indicated that they fold correctly (Fig. (a)). The thermal stability analysis using CD indicated that the analyzed proteins exist in the folded state at a temperature below 40 °C (data not shown). The AUC-SV experiments for CBM-DK wild-type and D270A/W273A/G358W showed two peaks: a main peak (13.8 kDa for wild-type, 14.4 kDa for mutant) and a minor peak (27.1 kDa for wild-type, 25.6 kDa for mutant), corresponding to the expected molecular masses of monomers (theoretical value, 15.5 kDa) and dimers (theoretical value, 31.1 kDa), respectively (Fig. (b)). The results indicate that CBM-DK molecules exist mainly in a monomeric state (~70%). Taken together, all the CBM-DK mutant proteins showed tertiary structures and folded states, similar to those of the wild-type.
Fig. 2. Secondary structure and monomeric state analyses. (a) Far-UV CD spectra of CBM-DK wild-type (solid line), D270A/W273A/D314W (dotted line), and D270A/W273A/G358W (broken line). The spectra of other mutants used in this study were also similar. (b) (upper) Sedimentation pattern and best fit result obtained from the c(s) analysis of a solution of CBM-DK wild-type and D270A/W273A/G358W. Residuals between observed data and best-fit result are also indicated. (lower) Distribution of CBM-DK wild-type and D270A/W273A/G358W.
The interactions of the Trp-switched mutants with laminarioligosaccharides and laminarin were analyzed using SPR biosensor, Biacore (Fig. ). The binding affinities determined from Scatchard analysis are summarized in Table . As reported previously,Citation9) CBM-DK interacts with laminarioligosaccharides in a monovalent manner, while it interacts with laminarin in a multivalent manner. In Scatchard plots for laminarin binding, the different binding modes resulted in a gradual increase in the slope at a lower occupancy. Therefore, the Ka values were determined from a linear fit in a concentration range of 5–40 and 0.625–5 μM of laminarin, and these values were compared between the CBM-DK molecules (Table ). The binding affinities of D270A, D270A/W273A, and W273A mutants to both laminarioligosaccharides and laminarin were either lost or much lower than that of the wild-type. Mutants with Trp introduced in their β- and γ-repeats showed higher binding affinities than those of W273A and D270A/W273A mutants. Based on the observation that β-glucan binding affinity of D270A/W273A mutant is under the detection limit of Biacore, we confirmed that the β-glucan mainly binds to the Trp-introduced site. The Trp-introduced repeat bound monovalently to laminarioligosaccharides, and primarily bound to laminarin, with additional contribution from other repeats.
Fig. 3. Typical sensorgrams for the binding of laminarin to D270A/W273A/D314W. (a) D270A/W273A/D314W was immobilized on a CM5 sensorchip and laminarin (0.625–40 μM) was flowed over the chip surface at rate of 20 μl/min for 3 min, followed by 3 min dissociation. (b) Scatchard plots for the laminarin binding. The Ka values determined from the linear fitting to the laminarin in the range of 5–40 μM and 0.625–5 μM, respectively.
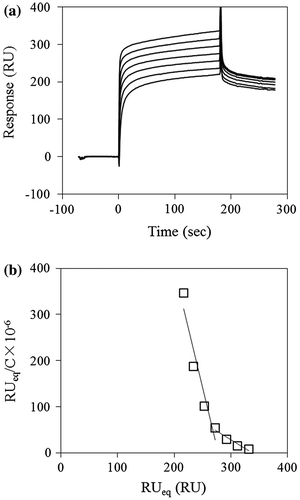
Table 1. Ka values (M−1) for interactions of β-glucans with immobilized CBM-DK and its mutants on the sensor chip.
The laminarin binding was also evaluated using ITC (Fig. ). The Ka values obtained from ITC experiments were mainly derived from interactions of single CBM-DK molecules, while those obtained from Biacore were derived from the interactions of multiple CBM-DK molecules immobilized on the sensor chip.Citation9) Similar to the results of Biacore, the binding affinities recovered in Trp-switched mutants, in comparison with those of D270A, D270A/W273A, and W273A mutants (Table ). All mutants showed similar values of binding stoichiometry, laminarin/CBM-DK ≈ 0.12, indicating that approximately 8 CBM-DK molecules bind to a single laminarin molecule. Together with the Biacore data, the Ka value in case of laminarin was higher than that in case of laminarioligosaccharides, indicating that multiple binding sites of a single CBM-DK molecule are involved in laminarin binding, and the Trp-introduced repeat primarily contributes to the binding. In comparison with CBM-DK wild-type, the Trp-introduced mutants, W273A/D314W, W273A/G358W, D270A/W273A/D314W, and D270A/W273A/G358W, showed similar binding affinities toward laminarin but reduced binding affinities toward laminarioligosacchrides (Tables and ), indicating that Trp273-based α-repeat binding is more effective than that of β- and γ-repeats, bearing Trp314 and Trp358 mutations, respectively. Finally, the effects of D314W and G358W mutations on CBM-DK wild-type, without mutations in α-repeat, showed little improvements on the laminarin binding relative to the wild-type (Table ).
Fig. 4. Typical ITC data for the binding of laminarin to D270A/W273A/D314W. (a) The 500 μM laminarin solution was injected into the 100 μM D270A/W273A/D314W solution. (b) The data points were obtained by integration of the peaks in (a), corrected for the dilution heat, and plotted against the molar ratio, laminarin to D270A/W273A/D314W. The data were fitted using a nonlinear least-squares method.
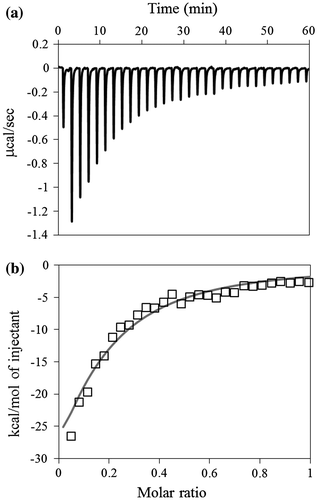
Table 2. Thermodynamic parameters of laminarin binding to CBM-DK and its mutants.
Discussion
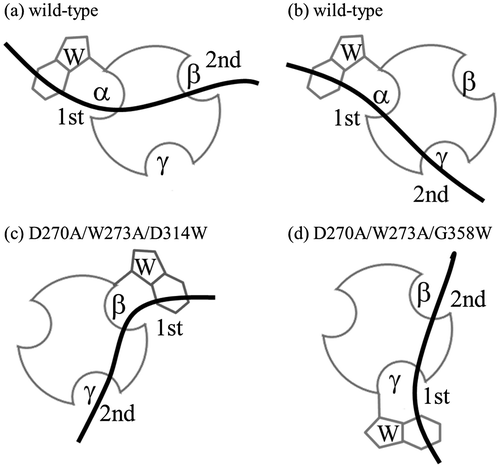
CBM-DK has a unique property that the α-repeat primarily contributes to β-glucan binding and the other two repeats bind it in a cooperative manner, resulting in binding laminarin with higher strength.Citation9) The different contribution of each repeat to β-glucan binding was hypothesized to be an effect of the hydrophobic contact derived from Trp. Our previous study also showed that W273S mutation reduced the β-glucan binding ability.Citation9) In this study, we focused on the role of Trp and generated new CBMs, which can interact with β-glucans primarily through β- and γ- repeats. These CBMs were engineered by switching the corresponding sites of Trp273 in β- and γ- repeats, residues 314 and 358, respectively, with Trp. The binding affinities of Trp-introduced mutants were clearly higher than that of the W273A mutant itself (Tables and ). It should be noted that Trp introduction in D270A/W273A mutant recovered its β-glucan binding ability. The binding affinities of Trp-introduced mutants to laminarin are higher than those to laminarioligosaccharides, similar to the case of CBM-DK wild-type (Table ). The W273A or D270A/W273A mutation reduced or eliminated the binding ability of α-repeat: introduction of Trp into β-repeat resulted in binding to laminarin primarily through the β-repeat, with additional contribution from α- or γ-repeat. Similarly, the mutant with Trp introduced into γ-repeat bound to laminarin primarily through γ-repeat with additional contribution from α- or β-repeat. Assuming that the Asp residues, Asp270 in α-repeat, Asp311 in β-repeat, and Asp355 in γ-repeat, also play a critical role in β-glucan binding,Citation9), we hypothesized that the γ-repeat would contribute to the additional binding in D270A/W273A/D314W mutant, and the β-repeat would contribute to the additional binding in D270A/W273A/G358W mutant (Fig. ). The binding thermodynamics showed that the Trp-introduced W273A mutants, W273A/D314W and W273A/G358W, have relatively less favorable ΔH and more favorable ΔS, in comparison with the Trp-introduced D270A/W273A mutants, D270A/W273A/D314W and D270A/W273A/G358W, whose binding thermodynamics are comparable to those of the wild-type (Table ). The hydrophobic contact with dehydration on Trp introduction possibly contributes to the different binding thermodynamics.
Fig. 5. Schematic representation of binding of CBM-DK wild-type (a, b) and its mutants, D270A/W273A/D314W (c) and D270A/W273A/G358W (d), to laminarin. The curved bold bars and circles with hollows represent laminarin and CBM-DKs, respectively. The Trp-introduced sites and the respective repeats are also indicated. The 1st and 2nd indicate the primary and secondary binding sites for laminarin.
The Trp-introduced mutants, which lacked Trp273, could monovalently bind to laminarioligosaccharides, but with reduced affinities than those of the wild-type. While the monovalent binding ability of Trp-introduced mutants is relatively low, the multivalent binding ability, in contrast, is comparable to that of the wild-type. This phenomenon seems to be similar to binding avidity of an antibody. The antigen binding affinity of germline-type is lower than that of affinity-matured-type, but the antigen binding avidity, multivalent binding affinity, derived from two Fab arms is comparable.Citation16) In addition, the Ka value around 106 M−1 might be the saturation value of binding affinity, as observed in case of an antibody.Citation17) In the Trp-introduced mutants, D314W and G358W, the binding abilities were similar to that of the wild-type (Table ). There might be potential maxima of available β-glucans because of the CBM-DK architecture. A single repeat preference toward the β-glucans might be reasonable to achieve the maximum binding affinity. Furthermore, it can be considered that the α-repeat preference of CBM-DK is reasonable, based on its architecture. This is supported by the results that W273A/D314W and W273A/G358W mutants have lesser binding affinities to laminarioligosaccharides, as compared to those of the wild-type (Table ). Based on our assumption that Pro272 in α-repeat orients Trp273 better for β-glucan binding (Fig. ), we also introduced Pro at the corresponding sites in β- and γ-repeats, generating W273A/R313P/D314W and W273A/T357P/G358W mutants, and analyzed their β-glucan binding. The results showed little improvements in binding affinities (data not shown).
Our previous study on endo-1,3-β-glucanase from Cellulosimicrobium cellulans DK-1 and its isolated catalytic domain showed that the presence of CBM-DK could enhance the stability of the catalytic domain at an acidic pH,Citation7) possibly due to the intramolecular interaction between the catalytic domain and CBM-DK. Although there is no direct observation of an intramolecular interaction, the α-repeat preference for β-glucan binding might be related to it. It can be expected that α-repeat can assist hydrolysis through the catalytic domain, even when CBM-DK interacts with the catalytic domain. CBMs from family 13 are considered to assist localization of catalytic domains with insoluble polysaccharides,Citation18,19) and CBM-DK contributes to increased activity of the full-length enzyme for curdlan and yeast-glucan.Citation7) The binding preference of α-repeat might be sufficient for the substrate-binding strength or the binding kinetics, which is efficient for the hydrolysis by the catalytic domain. The analysis for relative orientation of catalytic domain and CBM-DK is under progress using small angle X-ray scattering, which can clarify the preferential choice of α-repeat, over β- or γ-repeat, for β-glucan binding.
Author contributions
MO designed the research, AM prepared samples and carried out CD, SPR, and ITC, TM and YK carried out AUC, SI and MO wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Cooperative Research Program of Institute for Protein Research, Osaka University, CR-15-06.
Acknowledgments
The authors thank Tomonari Tamashiro and Hiromi Asada of Kyoto Prefectural University for technical support. The authors also thank Editage (www.editage.jp) for English language editing.
References
- http://www.cazy.org/Carbohydrate-Binding-Modules.html
- Gilbert HJ, Knox JP, Boraston AB. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr Opin Struct Biol. 2013;23:669–677.10.1016/j.sbi.2013.05.005
- Lu H, Luo H, Shi P, et al. A novel thermophilic endo-β-1,4-mannanase from Aspergillus nidulans XZ3: functional roles of carbohydrate-binding module and Thr/Ser-rich linker region. Appl Microbiol Biotechnol. 2014;98:2155–2163.10.1007/s00253-013-5112-6
- Rosa AM, Louro AF, Martins SA, et al. Capture and detection of DNA hybrids on paper via the anchoring of antibodies with fusions of carbohydrate binding modules and ZZ-domains. Anal Chem. 2014;86:4340–4347.10.1021/ac5001288
- Oliveira C, Carvalho V, Domingues L, et al. Recombinant CBM-fusion technology – Applications overview. Biotechnol Adv. 2015;33:358–369.10.1016/j.biotechadv.2015.02.006
- Tanabe Y, Pang Z, Oda M. Cloning and sequencing of endo-1,3-β-glucanase from Cellulosimicrobium cellulans. J Biol Macromol. 2008;8:60–63.
- Tanabe Y, Oda M. Molecular characterization of endo-1,3-β-glucanase from Cellulosimicrobium cellulans: effects of carbohydrate-binding module on enzymatic function and stability. Biochim Biophys Acta – Proteins Proteomics. 2011;1814:1713–1719.10.1016/j.bbapap.2011.09.004
- Boraston AB, Tomme P, Amandoron EA, et al. A novel mechanism of xylan binding by a lectin-like module from Streptomyces lividans xylanase 10A. Biochem J. 2000;350:933–941.10.1042/bj3500933
- Tamashiro T, Tanabe Y, Ikura T, et al. Critical roles of Asp270 and Trp273 in the α-repeat of the carbohydrate-binding module of endo-1,3-β-glucanase for laminarin-binding avidity. Glycoconj J. 2012;29:77–85.10.1007/s10719-011-9366-x
- Notenboom V, Boraston AB, Williams SJ, et al. High-resolution crystal structures of the lectin-like xylan binding domain from Streptomyces lividans xylanase 10A with bound substrates reveal a novel mode of xylan binding. Biochemistry. 2002;41:4246–4254.10.1021/bi015865j
- Fujimoto Z. Structure and function of carbohydrate-binding module families 13 and 42 of glycoside hydrolases, comprising a β-trefoil fold. Biosci Biotechnol Biochem. 2013;77:1363–1371.10.1271/bbb.130183
- Schärpf M, Connelly GP, Lee GM, et al. Site-specific characterization of the association of xylooligosaccharides with the CBM13 lectin-like xylan binding domain from Streptomyces lividans xylanase 10A by NMR spectroscopy. Biochemistry. 2002;41:4255–4263.10.1021/bi015866b
- Inaba S, Fukada H, Ikegami T, et al. Thermodynamic effects of multiple protein conformations on stability and DNA binding. Arch Biochem Biophys. 2013;537:225–232.10.1016/j.abb.2013.07.014
- Sato Y, Tanaka Y, Inaba S, et al. Structural dynamics of a single-chain Fv antibody against (4-hydroxy-3-nitrophenyl)acetyl. Int J Biol Macromol. 2016;91:151–157.10.1016/j.ijbiomac.2016.05.074
- Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys J. 2000;78:1606–1619.10.1016/S0006-3495(00)76713-0
- Oda M, Azuma T. Reevaluation of stoichiometry and affinity/avidity in interactions between anti-hapten antibodies and mono- or multi-valent antigens. Mol Immunol. 2000;37:1111–1122.10.1016/S0161-5890(01)00028-1
- Foote J, Eisen HN. Kinetic and affinity limits on antibodies produced during immune responses. Proc Nat Acad Sci USA. 1995;92:1254–1256.10.1073/pnas.92.5.1254
- Ferrer P. Revisiting the Cellulosimicrobium cellulans yeast-lytic β-1,3-glucanases toolbox: a review. Microb Cell Fact. 2006;5:10.10.1186/1475-2859-5-10
- Li N, Shi P, Yang P, et al. A xylanase with high pH stability from Streptomyces sp. S27 and its carbohydrate-binding module with/without linker-region-truncated versions. Appl Microbiol Biotechnol. 2009;83:99–107.
