Abstract
The multiple physiological effects of γ-aminobutyric acid (GABA) as a functional food component have been recently reported. We previously reported that GABA upregulated the expression of type I collagen in human dermal fibroblasts (HDFs), and that oral administration of GABA significantly increased skin elasticity. However, details of the regulatory mechanism still remain unknown. In this study, we further examined the effects of GABA on elastin synthesis and elastin fiber formation in HDFs. Real-time PCR indicated that GABA significantly increased the expression of tropoelastin transcript in a dose-dependent manner. Additionally, the expression of fibrillin-1, fibrillin-2, and fibulin-5/DANCE, but not lysyl oxidase and latent transforming factor-β-binding protein 4, were also significantly increased in HDFs. Finally, immunohistochemical analysis confirmed that treatment with GABA dramatically increased the formation of elastic fibers in HDFs. Taken together, our results showed that GABA improves skin elasticity in HDFs by upregulating elastin synthesis and elastin fiber formation.
The skin is the largest organ of the human body, and functions primarily as a protective barrier against environmental insults, such as ultraviolet irradiation, bacterial infection, mechanical and/or chemical stress, injury, heat and water loss. The skin is composed of two primary layers, the epidermis and dermis, and each layer performs specific physiological functions. The barrier function of the skin is exclusively achieved by the epidermis, which directly faces the external environment. Meanwhile, the dermis, which is located below the epidermis, maintains the skin’s strength, elasticity, and moisture.Citation1)
In the dermis, human dermal fibroblasts (HDFs) are responsible for the production of extracellular matrix (ECM) proteins, such as type I collagen, elastin, and proteoglycans. The ECM is a large component of the dermis, and is critical for maintaining the normal physiological structure of the skin. Type I collagen is the most abundant ECM protein of the skin’s connective tissue, and newly synthesized type I collagen from HDFs results in the formation of collagen fibril bundles, which are responsible for the strength and resiliency of the skin.Citation2) In addition to collagen fibrils, elastic fibers are also essential ECM components of the dermal connective tissue. Elastic fibers are composed mainly of two morphologically distinct components, elastin and microfibrils. Elastin is comprised of tropoelastin proteins crosslinked with lysyl oxidase. The crosslinked elastin molecules associate with the microfibrils, and are then formed into the elastic fibers. The elastic fibers, together with the collagen bundles, form the fibrillar network within the dermis, providing strength, extensibility and elasticity to the skin.Citation3)
The amino acid γ-aminobutyric acid (GABA) is widely distributed in plants, microorganisms, and animals, and is synthesized by the decarboxylation of glutamate. In mammals, GABA acts as the major inhibitory neurotransmitter in the central nervous system.Citation4) We previously reported that the administration of GABA produced microbially improved atopic dermatitis-like skin lesions in NC/Nga mice by adjusting the Th1/Th2 balance.Citation5) Furthermore, a placebo-controlled, double-blind study indicated that the oral administration of biosynthesized GABA significantly increased skin elasticity.Citation6) Biosynthesized GABA administration upregulated the expression of type I collagen and downregulated matrix metalloproteinase (MMP)-1 expression in HDFs in vitro.Citation7)
However, detailed knowledge of its regulatory mechanism is lacking, especially regarding the control of elastic fiber formation. Thus, in this study, we investigated the in vitro effects of GABA produced microbially and chemically on elastin synthesis and elastic fiber formation in HDFs to confirm whether the effects can be attributed to GABA alone. Elastin is the main component of elastic fibers, and is encoded by a single gene. Elastin is an insoluble crosslinked polymer assembled with tropoelastin, a soluble monomer precursor that is secreted by HDFs. Besides elastin, microfibrils are another important component of elastic fibers. The composition of microfibrils is more complex than elastin, with fibrillins (large cysteine-rich glycoproteins) being the major structural elements. Fibrillins are a family of three molecules, fibrillin-1, fibrillin-2, and fibrillin-3, which are encoded by distinct genes. Fibrillin-1 and fibrillin-2 have overlapping patterns of expression, and may be particularly important in elastic fiber formation. In contrast, fibrillin-3 was isolated from the brain, and its role in elastic fiber formation remains to be clarified. And tropoelastin is composed of lysine-containing hydrophobic crosslinking domains, as described above. In the process of elastic fiber formation, lysyl oxidase plays a critical role in the catalysis of lysine-derived crosslinks of elastin in dermal ECM. Other microfibril proteins are also important in the formation of elastic fibers in dermal ECM. Fibulin is one of the components of microfibrils, and fibulin-5, also known as DANCE, plays a role in the stabilization and organization of elastic fibers in the skin. In addition to microfibrils, several microfibril-associated proteins have been implicated as critical molecules in elastogenesis. Among them, latent transforming factor-B-binding protein 4 (LTBP-4), which shares structural homology with fibrillins, is necessary for elastin assembly on microfibrils through the interaction with fibulin-5.
Materials and methods
Preparation of GABA
GABA produced chemically and microbially was used in this study. Chemically synthesized GABA (99% purity) was purchased from Sigma–Aldrich Japan LLC (Tokyo, Japan). Microbially produced GABA (92% purity) was obtained by natural fermentation using a lactic acid bacterium as described previously with some modificationsCitation5) from SANWA SHURUI Co., Ltd. (Oita, Japan).
The nitrogen content in the biosynthesized GABA was measured according to the Kjeldahl method, while the amino acid content was measured using a high-speed amino acid analyzer L-8800 (Hitachi High-Technologies Corporation, Tokyo, Japan). The constituents of the biosynthesized GABA are shown in Table .
Table 1. The constituents of the biosynthesized GABA.
Chemosynthesized and biosynthesized GABA was dissolved in phosphate buffered saline (PBS(-)).
Cells and cell culture
HDFs were purchased from Kurabo Industries Ltd (Osaka, Japan). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovin serum (FBS) in humidified 5% CO2 and 95% air. HDFs were plated into 24-well plates at a density of 4 × 104 cells/well for RNA analysis, or 96-well plates at a density of 5 × 103 cells/well for cell proliferation assay. The cells were cultured in DMEM with 0.5% FBS 24 h before treatment with GABA. The cells were further cultured with various concentrations of GABA for 24 h, and then the cells were collected for RNA analysis or cell proliferation assay.
Cell proliferation analysis
Cell viability was determined by using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s instruction. The absorbance of each well was measured at 450 nm.
RNA analysis
Total RNA was isolated from HDF using TRIzol Plus RNA Purification kit (Life Technologies, NY) according to the manufacturer’s instruction. Reverse transcriptions were carried out using SuperScriptIII First-strand Synthesis System (Life Technologies, NY) with Random primers, and the resulting single-stranded cDNA molecules were PCR amplified using gene-specific primers. For a quantification of mRNA, real-time PCR was performed using LightCycler Nano (Roche, Indianapolis, IN). The thermal cycler conditions included 1 cycle at 95 °C for 10 min, 45 cycles at 95 °C for 10 s, 60 °C for 10 s, 72 °C for 15 s. The relative mRNA expression levels were normalized against that of the GAPDH gene using a comparative threshold cycle method.Citation8) The sets of primers used in this study were described in Table .
Table 2. Primer sequences.
Immunohistochemistry analysis
HDFs were plated into 8-well chamber slide at a density of 16 × 103 cells/well for immunohistochemistry. The cells were cultured in DMEM with 0.5% FBS in the presence of 10 μg/mL GABA. After incubation with GABA for 7 days, the adherent cells on the slide were fixed with 10% formalin in PBS(-) for 15 min at RT. After washing with PBS(-), the cells were blocked with Blocking One Histo (Nacalai tesque, Osaka, Japan) for 5 min at RT. After washing with PBS(-), permeabilized with 0.1% Triton X-100 for 5 min at RT. After removing 0.1% Triton X-100, the cells were incubated with a polyclonal rabbit anti-human alpha-elastin (Bio-Rad, Ca, #4060-1060) for 1 h at RT. After washing with PBS(-), the cells were incubated with goat anti-rabbit IgG (H + L) Secondary antibody, Alexa Fluor 488 conjugate (Thermo Fisher, MA, #A-11008) for 1 h at RT. After washing with PBS(-), the cells were mounted with ProLong Gold Antifade Mountant with DAPI (4,6-diamidino-2-phenylinole), which is able to counterstain cell nuclei (Thermo Fisher, MA, #P-36935). The negative control was incubated with Blocking One Histo instead of the polyclonal rabbit anti-human alpha-elastin.
Statistical analysis
All results were presented as means ± SD of three independent experiments, and significant differences (p < 0.05) were evaluated by Tukey’s multiple comparison test.Citation9)
Results
Effect of GABA on the cell proliferation of HDFs
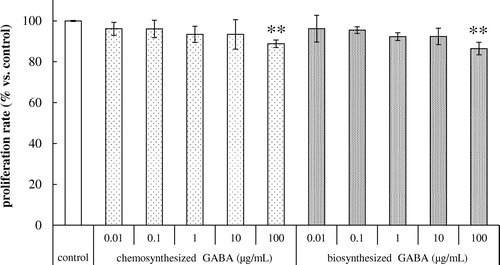
The cytotoxic effects of GABA on HDFs were evaluated using a cell proliferation assay. As shown in Fig. , both chemosynthesized and biosynthesized GABA at concentrations of 0.01, 0.1, 1 and 10 μg/mL had no cytotoxic effects on the proliferation of HDFs with incubation for 24 h. However, incubation with 100 μg/mL of chemosynthesized and biosynthesized GABA significantly decreased the cell viability of HDFs (Fig. ). Thus, 0.01–10 μg/mL of chemosynthesized and biosynthesized GABA was used in further experiments.
Fig. 1. Effects of GABA on the cell proliferation in HDFs. Cells were cultured for 24 h with various concentrations of chemosynthesized and biosynthesized GABA. Cell viability was determined by using Cell Counting Kit-8. Each datum represents the mean ± SD of three independent experiments. Results are compared to control, and significant difference is indicated by an asterisk (**p < 0.01).
Effect of GABA on tropoelastin mRNA expression in HDFs
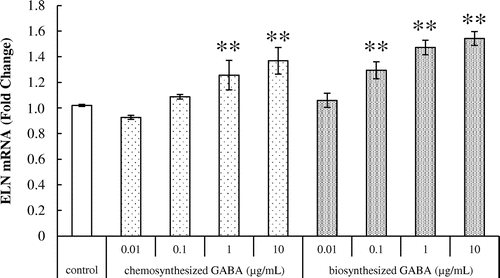
We next investigated the effect of GABA on tropoelastin mRNA expression in HDFs using real-time PCR. Results showed that both chemosynthesized and biosynthesized GABA significantly increased the expression of tropoelastin transcript in a dose-dependent manner (Fig. ), suggesting that GABA enhances the elastin production of HDFs.
Fig. 2. Effects of GABA on tropoelastin mRNA expression in HDFs. Cells were cultured for 24 h with various concentrations of chemosynthesized and biosynthesized GABA. The expression level of tropoelastin transcript was analyzed by real-time PCR. Each datum represents the mean ± SD of three independent experiments. Results are compared to control, and significant difference is indicated by an asterisk (**p < 0.01).
Effects of GABA on fibrillin-1 and fibrillin-2 mRNA expression in HDFs
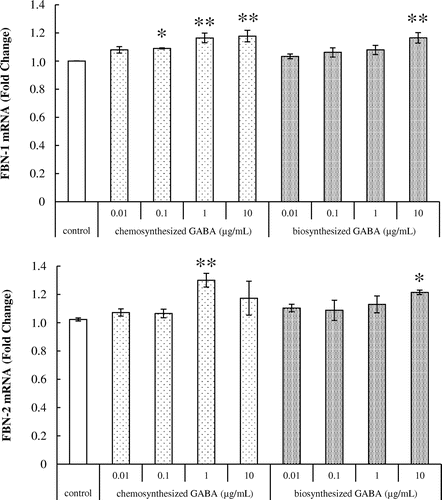
The effects of GABA on fibrillin-1 and fibrillin-2 mRNA expression in HDFs were examined. As shown in Fig. , chemosynthesized GABA significantly increased the expression of fibrillin-1 transcript in a dose-dependent manner. Treatment with 10 μg/mL of biosynthesized GABA significantly increased the mRNA expression of fibrillin-1. Fibrillin-2 was significantly increased in HDFs treated with 1 μg/mL of chemosynthesized GABA and 10 μg/mL of biosynthesized GABA. Taken together, these results suggest that GABA upregulates the production of elastic fiber components in HDFs.
Fig. 3. Effects of GABA on fibrillin-1 and fibrillin-2 mRNA expression in HDFs. Cells were cultured for 24 h with various concentrations of chemosynthesized and biosynthesized GABA. The expression level of fibrillin-1 and fibrillin-2 transcript was analyzed by real-time PCR. Each datum represents the mean ± SD of three independent experiments. Results are compared to control, and significant difference is indicated by an asterisk (*p < 0.05, **p < 0.01).
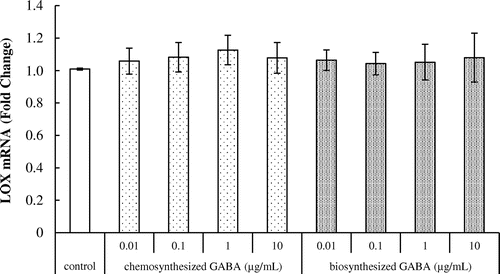
Effect of GABA on lysyl oxidase mRNA expression in HDFs
We examined the effect of GABA on lysyl oxidase mRNA expression in HDFs. As shown in Fig. , both chemosynthesized and biosynthesized GABA did not affect the expression of lysyl oxidase. This result indicates that GABA does not influence the crosslinked polymer assembly of tropoelastin.
Fig. 4. Effects of GABA on lysyl oxidase mRNA expression in HDFs. Cells were cultured for 24 h with various concentrations of chemosynthesized and biosynthesized GABA. The expression level of lysyl oxidase transcript was analyzed by real-time PCR. Each datum represents the mean ± SD of three independent experiments. Results are compared to control.
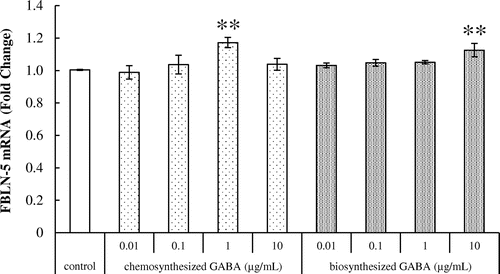
Effects of GABA on fibulin-5 and LTBP-4 mRNA expression in HDFs
We further examined the effect of GABA on fibulin-5 mRNA expression in HDFs. As shown in Fig. , treatment with 1 μg/mL of chemosynthesized GABA and 10 μg/mL of biosynthesized GABA significantly increased the expression of fibulin-5 transcript.
Fig. 5. Effects of GABA on fibulin-5 mRNA expression in HDFs. Cells were cultured for 24 h with various concentrations of chemosynthesized and biosynthesized GABA. The expression level of fibulin-5 transcript was analyzed by real-time PCR. Each datum represents the mean ± SD of three independent experiments. Results are compared to control, and significant difference is indicated by an asterisk (**p < 0.01).
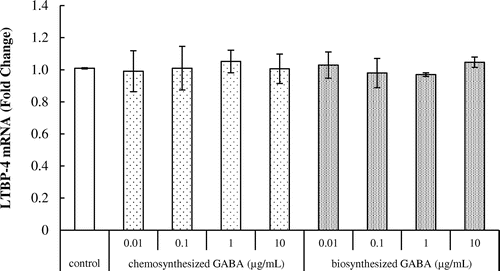
As shown in Fig. , however, GABA did not affect the expression of LTBP-4 transcript in HDFs.
Fig. 6. Effects of GABA on LTBP-4 mRNA expression in HDFs. Cells were cultured for 24 h with various concentrations of chemosynthesized and biosynthesized GABA. The expression level of LTBP-4 transcript was analyzed by real-time PCR. Each datum represents the mean ± SD of three independent experiments. Results are compared to control.
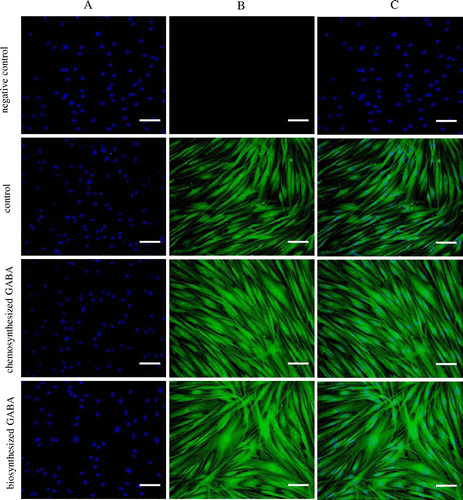
Effect of GABA on elastic fiber formation in HDFs
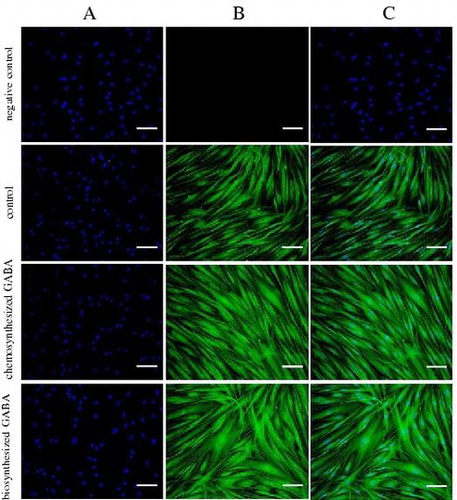
Finally, immunohistochemical analysis was conducted to confirm whether GABA promotes elastic fiber formation in HDFs. As shown in Fig. , in the negative control, staining of nuclei but not elastin was detected. Treatment with 10 μg/mL of both chemosynthesized and biosynthesized GABA dramatically increased the formation of elastic fibers in HDFs. Thus, collectively these data indicate that GABA enhances the production of elastic fiber components, such as tropoelastin and fibrillins, and subsequently promotes elastin fiber formation in HDFs.
Fig. 7. GABA enhanced the elastic fiber formation in HDFs. Cells were cultured for 7 days with 10 μg/mL of chemosynthesized and biosynthesized GABA. The negative control was determined using only the secondary antibody. Cells were stained with DAPI for nuclear counterstaining (A), with anti-human alpha-elastin antibody for elastic fibers (B), and merged (C). Scale bar, 100 μm.
Discussion
The aging process of skin is affected by many biological phenomena, and can be categorized separately as intrinsic and extrinsic aging. Intrinsic aging can be defined as the genetic regulation of skin homeostasis, such as changes in the endocrine environment, in a manner similar to other internal organs, while extrinsic aging is a result of environmental influences, such as ultraviolet radiation and pollutants.Citation10) Both types of skin aging are induced by the breakdown of skin ECM proteins, which are mainly composed of collagen fibrils and elastic fibers. Collagen fibrils are a major component of the dermis, which comprises approximately 70–80% by dry weight of the skin, and provides both mechanical and structural integrity to the dermis. On the other hand, while elastic fibers are a minor component, approximately 2–4% of the skin ECM, they play a critical role in skin elasticity.Citation11) During skin aging, collagen synthesis is gradually reduced, resulting in the disorganization of the collagen network. Furthermore, the production of elastic fibers is also reduced, resulting in impaired elasticity. HDFs are responsible for the production of ECM proteins, and play an important role in maintaining the skin’s normal physiological structure and function.Citation12) To maintain the structural integrity and elasticity of the skin, therefore, it is necessary to regulate the function of HDFs and the subsequent ECM protein expression.
On the other hand, functional micronutrients and food components can mitigate skin aging through anti-oxidant and/or anti-inflammatory effects.Citation13) In fact, several antioxidant compounds, such as vitamins and polyphenols, improved human skin elasticity by regulating the expression of collagen, MMPs and several components of elastic fibers.Citation14,15) Meanwhile, the amino acid GABA is found in many kinds of foods, especially fermented food products.Citation16) In trials involving healthy human subjects, GABA was rapidly absorbed and reached a peak concentration in the plasma 1 h after oral administration, resulting in significantly increased circulating insulin levels to improve diabetes.Citation17)
We also previously reported that oral administration of biosynthesized GABA significantly increased skin elasticity,Citation6) and treatment with biosynthesized GABA significantly upregulated the expression of type I collagen and downregulated MMP-1 expression in HDFs.Citation7) In this study, we further examined whether GABA regulates elastic fiber formation in HDFs. Elastic fibers are a major component of the dermal ECM, and consist of two morphologically distinct components. The major component is elastin, which is comprised of crosslinked tropoelastin, while the other is fibrillin-rich microfibrils, which consist of fibrillin-1 and fibrillin-2. The expression of both components is essential for the maintenance of skin elasticity and flexibility. For example, exposure to UV radiation reduced the levels of elastin and fibrillin, resulting in the induction of wrinkles and the loss of elasticity.Citation3) In an experimental mouse model, mutation in the fibrillin-1 and/or fibrillin-2 genes impaired or delayed elastogenesis.Citation18) In this study, as shown in Figs. and , treatment with GABA significantly increased the expression of elastin and fibrillin in HDFs, suggesting that GABA improves skin elasticity and flexibility through the upregulation of elastic fiber synthesis. In addition to elastin and fibrillin, other molecules are also involved in the formation of elastic fibers in the dermal ECM. Among these, fibulin-5, a component of microfibrils, is essential for the production of elastic fibers, as evidenced by the abnormalities observed in elastic fibers in the dermis of fibulin-5 null miceCitation19) and the acceleration of elastic fiber assembly in HDFs by fibulin-5.Citation20) In this study, GABA also increased the expression of fibulin-5, suggesting that GABA enhances elastic fiber assembly in HDFs. Finally, immunohistochemical analysis confirmed that GABA enhanced elastic fiber formation in HDFs (Fig. ). Taken together, our data suggest that GABA promotes elastin synthesis and elastic fiber formation in HDFs.
In this study, we additionally used both chemosynthesized and biosynthesized GABA to confirm whether the observed in vitro effects can be attributed to GABA alone. Since both the chemosynthesized and microbially produced GABA showed the same effects on mRNA expression and immunohistochemical observations, GABA was proposed as the primary mediator of the effect.
In conclusion, we demonstrated that GABA increases the expression of tropoelastin, fibrillin-1, fibrillin-2, and fibulin-5, resulting in enhanced elastic fiber formation. Thus, our study suggests that GABA administration improves fiber elasticity in HDFs by promoting the production of elastic fiber components, resulting in enhanced elastic fiber formation, and by controlling the balance between type I collagen synthesis and degradation. GABA has obvious utility as a functional food component for the improvement of skin elasticity.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
Abbreviations: GABA; γ-aminobutyric acid;HDFs; human dermal fibroblasts;LTBP-4; latent transforming factor-β-binding protein 4
References
- Naylor EC, Watson REB, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;69:249–256.10.1016/j.maturitas.2011.04.011
- Callaghan TM, Wilhelm KP. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: cellular and molecular perspectives of skin ageing. Int J Cosmetic Sci. 2008;30:313–322.10.1111/ics.2008.30.issue-5
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828.
- Erdö SL. Peripheral GABAergic mechanisms. Trends Pharmacol Sci. 1985;6:205–208.10.1016/0165-6147(85)90096-3
- Hokazono H, Omori T, Ono K. Effects of single and combined administration of fermented barley extract and γ-aminobutyric acid on the development of atopic dermatitis in NC/Nga mice. Biosci Biotechnol Biochem. 2010;74:135–139.10.1271/bbb.90653
- Hokazono H, Uehara E. Dermal effects of oral administration of GABA in humans. J Jpn Soc Food Sci Technol. 2016;63:306–311.10.3136/nskkk.63.306
- Uehara E, Hokazono H, Sasaki T, et al. Effects of GABA on the expression of type I collagen gene in normal human dermal fibroblasts. Biosci Biotechnol Biochem. 2017;81:376–379.10.1080/09168451.2016.1238296
- Page PB, Stromberg AJ. Linear methods for analysis and quality control of relative expression ratios from quantitative real-time polymerase chain reaction experiments. Sci World J. 2011;11:1383–1393.10.1100/tsw.2011.124
- Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol. 1998;25:1032–1037.10.1111/cep.1998.25.issue-12
- Yadav T, Mishra S, Das S, et al. Anticedants and natural prevention of environmental toxicants induced accelerated aging of skin. Envirn Toxicol Phamacol. 2015;39:384–391.10.1016/j.etap.2014.11.003
- Uitto J. Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. J Invest Dermatol. 1979;72:1–10.10.1111/1523-1747.ep12530093
- Tzaphidou M. The role of collagen and elastin in aged skin: an image processing approach. Micron. 2004;35:173–177.10.1016/j.micron.2003.11.003
- Park K. Pole of micronutrients in skin health and function. Biomol Ther. 2015;23:207–217.10.4062/biomolther.2015.003
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8–16.10.15280/jlm.2014.4.1.8
- Zillich OV, Schweiggert-Weisz U, Eisner P, et al. Polyphenols as active ingredients for cosmetic products. Int J Cosmetic Sci. 2015;37:455–464.10.1111/ics.2015.37.issue-5
- Komatsuzaki N, Shima J, Kawamoto S, et al. Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 2005;22:497–504.10.1016/j.fm.2005.01.002
- Li J, Zhang Z, Liu Z, et al. Study of GABA in healthy volunteers: pharmacokinetics and pharmacodynamics. Front Phamacol. 2015;6: Article 260.
- Carta L, Pereira L, Artega-Solis SE, et al. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem. 2006;281:8016–8023.10.1074/jbc.M511599200
- Nakamura T, Lozano PR, Ikeda Y, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175.10.1038/415171a
- Katsuta Y, Ogura Y, Iriyama S, et al. Fibulin-5 accelerates elastic fibre assembly in human skin fibroblasts. Exp Dermatol. 2008;17:837–842.10.1111/exd.2008.17.issue-10
