Abstract
Enzymatically prepared alginate oligomer (AO) promoted the growth of Chlamydomonas reinhardtii in a concentration-dependent manner. AO at 2.5 mg/mL induced increase in expression levels of cyclin A, cyclin B, and cyclin D in C. reinhardtii. CuSO4 at 100 μM suppressed the growth of C. reinhardtiin, and AO at 2.5 mg/mL significantly alleviated the toxicity of CuSO4. Increased intracellular reactive oxygen species level in C. reinhardtii induced by CuSO4 was reduced by AO. After cultivation with CuSO4 at 100 μM, expression levels of ascorbate peroxidase and superoxide dismutase in C. reinhardtii were increased, and AO reduced the increased levels of these enzymes. These results suggest that AO exhibits beneficial effects on C. reinhardtii through influencing the expression of various genes not only at normal growth condition but also under CuSO4 stress.
Alginate is an acidic linear polysaccharide found in certain brown algae such as Ascophyllum nodosum and Laminaria japonica, and is constituted of α-l-guluronate and β-d-mannuronate. The residues are arranged in a block structure of a homopolymer (polyguluronate or polymannuronate) or heteropolymer (a mixed sequence of these residues).Citation1) Alginate has some useful physicochemical characteristics such as high viscosity in aqueous solution and gel-forming property in the presence of divalent cations such as calcium ion. Alginate shows various biological activities in mammalian systems. It stimulates immune cells such as macrophages to secrete interleukin-6 and tumor necrosis factor (TNF)-α,Citation2) and has antitumor activity.Citation3,4) Furthermore, previous studies have demonstrated that alginate polymer shows anti-diabetic effect,Citation5) inhibition of smooth-muscle cell proliferation,Citation6,7) and anti-allergic effect through inhibition of histamine release from mast cells.Citation8)
Alginate oligosaccharides (oligomers) with relatively low molecular weight prepared by enzymatic degradation of alginate polymers are also known to have several activities in various mammalian systems such as suppression of fibroblast proliferation and collagen synthesis in human skin,Citation9) stimulation of endothelial cell growth and migration,Citation10) and enhancement of human keratinocyte growth.Citation11) Our previous studies have reported that alginate oligomer prepared by alginate lyase induced secretion of higher levels of nitric oxide and TNF-α from mouse macrophage cell line RAW264.7 cells than those of original alginate polymer.Citation12,13) Recently, we have found that enzymatically prepared alginate oligomer showed anti-obesity effects in a high-fat diet mice model.Citation14) In addition to mammalian systems, there are some reports regarding the effects of alginate oligomer on higher plants. Alginate oligomer prepared with bacterial alginate lyase increased the shoot elongation of komatsuna (Brassica rapa var. pervidis) seeds,Citation15) and promoted the elongation of barley roots.Citation16)
Furthermore, recent studies have demonstrated that alginate oligomer exhibits beneficial effects on some species of microalgae as well. Microalgae inhabiting in fresh and marine water play an important role as primary feed in a food chain. In aquaculture industry, Nannochloropsis oculata and Chaetoceros gracilis are important marine microalgae used as feeding sources for bivalves and larvae of some crustacean and fish species, and these microalgae contain important nutrients such as vitamin E and polyunsaturated fatty acids which are essential for certain fish species. On the other hand, Botryococcus braunii and Aurantiochytrium sp. can store high concentration of hydrocarbon in intracellular compartments, and these microalgae are expected to be utilized as promising sources of biofuel.Citation17,18) Algal oil, biofuel, and biodiesel have become increasingly attractive subjects as global search for alternatives of fossil fuels. However, these microalgae often suffer from suddenly caused mass mortality due to unknown mechanisms. Hence, it is important to establish improved culture procedures for commercially valuable microalgae species. In our previous studies, alginate oligomer showed growth promoting effects on marine microalga N. oculataCitation19) and freshwater green microalga Chlamydomonas reinhardtii.Citation20) The growth promoting activity on C. reinhardtii was found in enzymatically digested alginate oligomer, but oligomer prepared by acid hydrolysis showed no such activity.Citation20) Hence, it seems that enzymatic preparation of alginate oligomer is a key to obtain the active alginate oligomer. C. reinhardtii occurs globally in many soil and freshwater environments. It is well recognized that C. reinhardtii is not only valuable model species for fundamental studies in plant physiology, biochemistry, and molecular biology, but also an important species for studies on algal hydrogen production.Citation21) Interestingly, cellular levels of some fatty acids in C. reinhardtii cultured with alginate oligomer increased significantly as compared to control levels,Citation20) suggesting that alginate oligomer could be used as a promoting agent for efficient fatty acid production by microalga as a source of biofuel. In addition, the toxic effect of heavy metal (i.e. CuSO4) on N. oculata was almost completely disappeared by the addition of alginate oligomer,Citation19) suggesting that alginate oligomer could be a potential alleviator against heavy metal stress. However, action mechanism of alginate oligomer on growth promoting effect and protective effect on CuSO4-induced toxicity in plant cells including microalgae such as C. reinhardtii is still unclear, and there is no analytical study on the effects of alginate oligomer on gene expression in C. reinhardtii. To gain insight into action mechanism of alginate oligomer on microalgae, in this study, we investigated its effect on gene expression levels of C. reinhardtii under normal and CuSO4-induced stress conditions.
Materials and methods
Reagents
Sodium alginate (1000-cps grade) was purchased from Nacalai Tesque Inc. (Kyoto, Japan). Alginate lyase was obtained from Nagase ChemteX Co. (Osaka, Japan). Carboxy-methyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) was purchased from Invitrogen (Eugene, OR, USA). All other chemicals were of the highest grade commercially available.
Preparation of alginate oligomer
Alginate oligomer (AO) was prepared from sodium alginate by the method described previously.Citation22) Alginate oligomer used in this study was abbreviated as AO hereafter. In brief, 1% of sodium alginate in ultra-pure water was incubated with alginate lyase (final concentration, 1 μg/mL) at 40 °C for three days. The enzymatic reaction was stopped by adding HCl to adjust pH 4.0, and the reaction mixture was incubated for 10 min, and then neutralized with NaOH. The reaction mixture was lyophilized with a vacuum freeze-dryer.
Chlamydomonas reinhardtii
C. reinhardtii (CC3395; arginine-requiring strain) was purchased from Chlamydomonas Resource Center (St. Paul, MN, USA), and cultured in 100-mL conical flasks containing 50 mL of Tris-acetate-phosphate (TAP) mediumCitation23) supplemented with filter-sterilized l-arginine at a final concentration of 100 μg/mL at 25 °C with continuous shaking (100 rpm/min) in the light (200 μmol photons/m2/s of cool-white fluorescence illumination). All of the cultures were handled with sterilized instruments.Citation20) C. reinhardtii cells in exponential growth phase were used throughout the experiments. To examine the effects of AO on the growth of C. reinhardtii, varying concentrations of AO were added to C. reinhardtii cell suspension at an initial cell density of 1 × 104 cells/mL in 48-well plate (1 mL/well), and cultured at 25 °C. After 4 days cultivation, the cell numbers in each well were counted microscopically with a hemocytometer (Erma, Tokyo, Japan) after fixation with 0.5% glutaraldehyde.
Gene expression analysis
To analyze gene expression, total RNA was extracted from C. reihardtii cells by acid guanidinium-phenol-chloroform (AGPC) method. C. reinhardtii cells cultured under various conditions were harvested by centrifugation at 760 x g for 10 min, and crushed in TRIsure (NIPPON Genetics Co., Ltd., Tokyo, Japan) with beads by Shake Master (Bio Medical Science Inc., Tokyo, Japan). The extracted total RNA (1 μg) was reverse transcribed into a single-stranded cDNA using a PrimeScript® 1 st strand cDNA Synthesis Kit (TaKaRa BioInc., Shiga, Japan). Quantitative polymerase chain reaction (PCR) was performed using SYBR® Select Master Mix (Applied Biosystems, Foster City, CA, USA), and analyzed by 7300 Real Time PCR System (Applied Biosystems). Quantitative PCR parameters for cycling were as follows: 1 cycle of 2 min at 50 °C, 95 °C for 2 min 40 cycles of PCR at 95 °C for 15 s, and 60 °C for 30 s. All the reactions were done in a 20 μL reaction volume in triplicate. The mRNA expression was determined using the 2-∆Ct method, and mRNA expression levels were normalized by using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an endogenous control. The primers used in this study were shown in Table .
Table 1. Sequences of primers used in this study for real time PCR analysis.
Effect of CuSO4
To examine the effects of copper ion on the growth of C. reinhardtii, the indicated final concentrations of CuSO4 (0–100 μM) and AO (0–10 mg/mL) were added to C. reinhardtii cell suspension in 48-well plate. After 6 days cultivation, the cell numbers in each well were counted.
Analysis of intracellular reactive oxygen species
For the analysis of intracellular reactive oxygen species (ROS) in C. reinhardtii cells, the cells in 96-well fluorescence black plate (3 × 106 cells/0.2 mL/well) were incubated with AO (final 0–10 mg/mL), CuSO4 (final 100 μM), and CM-H2DCFDA (final 10 μM) at 25 °C under cool-white fluorescence illumination. After 30 min incubation, fluorescence intensity in each well was measured with excitation wavelength at 490 nm and emission wavelength at 530 nm with Mithras LB940 (Berthold Technologies GmbH and Co. KG, Bad Wildbad, Germany).
Statistical analysis
All statistical analyses were performed using GraphPad prism 6 software (GraphPad Software, San Diego, CA, USA). A P-value of < 0.05 was considered as statistically significant.
Results
Effects of AO on the growth of C. reinhardtii
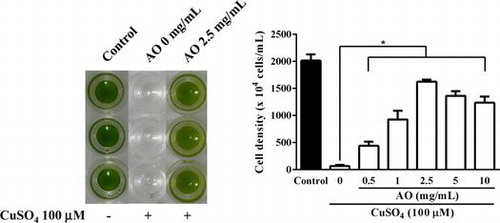
As shown in Fig. , addition of AO to the medium resulted in an increase in cell density of C. reinhardtii. The growth promoting effect of AO was concentration dependent with bell-shape profile, and the maximum effect was observed at 2.5 mg/mL. It seems likely that AO has an optimum concentration to promote the growth of C. reinhardtii. Interestingly, cell cycle related gene analysis by real time PCR showed that expression levels of cyclin A, B and D in C. reinhardtii treated with AO at 2.5 mg/mL were evidently higher than those of control (Fig. ). These results indicate that AO is capable of promoting the growth of C. reinhardtii through the stimulation of cell-cycle related gene expression.
Fig. 1. Effect of AO on the growth of C. reinhardtii. C. reinhardtii cells (1 × 104 cells/1 mL/well in 48-well plate) were incubated with indicated concentrations of AO for 4 days at 25 °C, and the cell numbers in each well were microscopically counted. Each value represents mean ± standard deviation of triplicate measurements. * indicate significant difference between with and without AO (p < 0.05).
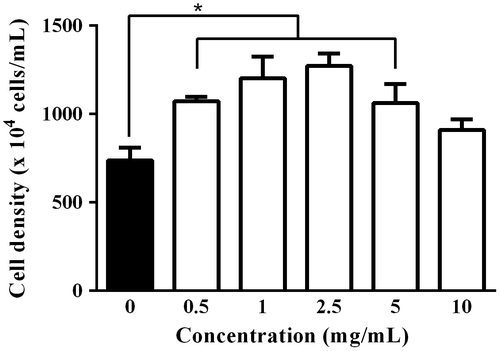
Fig. 2. Effect of AO on cell cycle related gene expression levels in C. reinhardtii. C. reinhardtii cells (2 × 106 cells/1 mL/well in 24-well plate) were incubated without (control; open column) or with 2.5 mg/mL of AO (solid column) for 24 h at 25 °C, and then cyclin A1 (CYCA1), cyclin B1 (CYCB1), and cyclin D1 (CYCD1) were analyzed by real time PCR as described in the text. GAPDH was used as an endogenous control. Each value represents mean ± standard deviation of triplicate measurements. * indicate significant difference between with and without AO (p < 0.05).
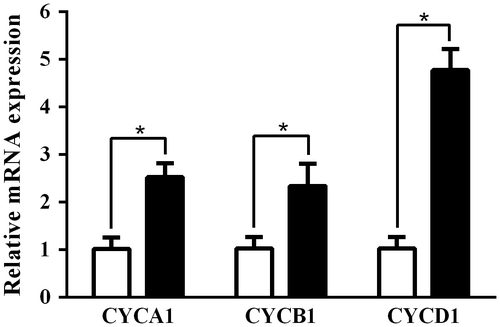
Fig. 3. Effect of AO on C. reinhardtii under copper ion stress. (A) C. reinhardtii cells (1 × 104 cells/1 mL/well in 48-well plate) were incubated with indicated concentrations of CuSO4 (0–100 μM) for 6 days at 25 °C, and the cell numbers in each well were microscopically counted. Each value represents mean ± standard deviation of quartette measurements. (B) C. reinhardtii cells (1 × 104 cells/1 mL/well in 48-well plate) were incubated with indicated concentrations of AO (0–10 mg/mL) in the presence of CuSO4 (100 μM) for 6 days at 25 °C, and then the cell numbers in each well were microscopically counted. Solid column was control cultured under normal condition. Each value represents mean ± standard deviation of triplicate measurements. * indicates significant difference between with and without AO (p < 0.05). (C) C. reinhardtii cells (1 × 104 cells/1 mL/well in 48-well plate) were cultured under normal condition (open circle), or with CuSO4 (100 μM) in the presence (semisolid circle) or absence of 2.5 mg/mL of AO (solid circle) for 6 days at 25 °C, and the cell numbers in each well were microscopically counted every day. Each value represents mean ± standard deviation of triplicate measurements.
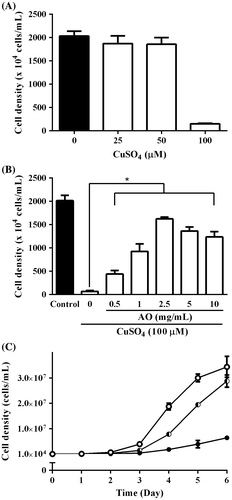
Fig. 4. Intracellular ROS levels in C. reinhardtii cells incubated under various conditions. C. reinhardtii cell suspensions containing 10 μM CM-H2DCFDA in 96-well fluorescence black plate (3 × 106 cells/0.2 mL/well) were incubated under normal condition (solid column) or with CuSO4 (100 μM) in the presence of varying concentrations of AO (0–10 mg/mL) (open column) for 30 min at 25 °C, and then fluorescence intensity of each well was measured with fluorescent microplate reader. Each value represents mean ± standard deviation of triplicate measurements. * indicates significant difference between with and without AO (p < 0.05).
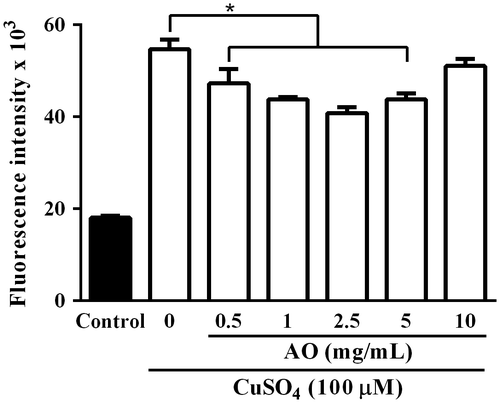
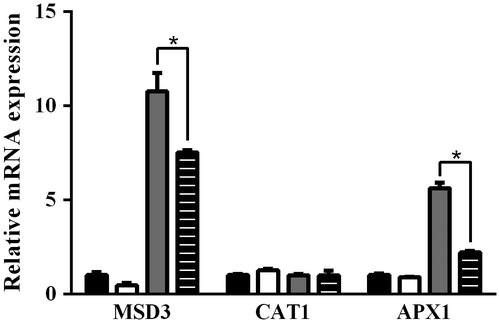
Fig. 5. Real time PCR analysis of expression levels of antioxidant enzymes in C. reinhardtii cells incubated under various conditions. C. reinhardtii cells in 24-well plate (3 × 106 cells/1 mL/well) were incubated under normal condition (open column) or with AO (2.5 mg/mL) alone (solid column), CuSO4 (100 μM) alone (shaded column), or CuSO4+AO (striped column) for 24 h at 25 °C, and then expression levels of manganese superoxide dismutase 3 (MSD3), catalase 1 (CAT1), and ascorbate peroxidase 1 (APX1) were analyzed by real time PCR. GAPDH was used as an endogenous control. Each value represents mean ± standard deviation of triplicate measurements. * indicate significant difference between with and without AO (p < 0.05).
Effects of CuSO4 on the growth of C. reinhardtii
To further examine the effect of AO on C. reinhardtii, we investigated effect of AO on C. reinhardtii under CuSO4 stress. As shown in Fig. (A), CuSO4 showed growth suppressive effect on C. reinhardtii, and the growth was almost completely inhibited at 100 μM. CuSO4-induced toxic effect on C. reinhardtii was alleviated with AO in a concentration-dependent manner (Fig. (B)), and the growth of C. reinhardtii was recovered up to nearly 80% of control by the addition of AO at 2.5 mg/mL even in the presence of 100 μM of CuSO4. At higher concentrations of AO (5 and 10 mg/mL), the protective effects were rather lower than at 2.5 mg/mL. Fig. (C) shows the growth curves of C. reinhardtii during 6 days cultivation under the various conditions. In the presence of AO at 2.5 mg/mL, the growth suppressed with CuSO4 was gradually recovered to control level during 6 days cultivation.
Intracellular ROS in C. reinhardtii
It has been reported that reactive oxygen species (ROS) is involved in copper ion stress in C. reinhardtii.Citation24) Hence, intracellular ROS level in C. reinhardtii cells in the presence of CuSO4 was investigated using ROS-specific fluorescence probe. As shown in Fig. , fluorescence intensity of cell suspension of C. reinhardtii incubated with CuSO4 (100 μM) for 30 min at 25 °C increased significantly as compared to control without CuSO4, and it was almost three times stronger than control. CuSO4-induced increase in the fluorescence intensity of C. reinhardtii cell suspension was suppressed by AO in a concentration-dependent manner, and the most effective suppression was observed at 2.5 mg/mL (Fig. ). These results suggest that AO can suppress CuSO4-induced increase in intracellular ROS level in C. reinhardtii cells. To gain insight into the mechanisms of suppressive effect of AO on CuSO4-induced increase in intracellular ROS production in C. reinhardtii cells, expression levels of anti-oxidative enzyme-related genes were examined by real time PCR (Fig. ). The results suggested that the expression levels of both MSD3 and APX1 but not CAT1 gene increased in C. reinhardtii cells incubated with CuSO4 (100 μM) for 24 h as compared to control, while adding of AO (2.5 mg/mL) resulted in reduction of both MSD3 and APX1 gene expression. On the other hand, AO alone showed no effect on the expression of these enzyme-related genes.
Discussion
Regarding the specificity of AO, the effects of enzymatically digested AO on the growth of various organisms have been investigated at cellular level using six mammalian cell lines (HeLa, Vero, MDCK, XC, CHO, and L929), two species of phytoplankton (C. gracilis and Skeletonema sp.), and two strains of marine bacteria (Edwardsiella tarda). Growth promoting effect of AO was observed in only diatom C. gracilis among the organisms tested.Citation22) These results suggest that AO preferably tends to show certain beneficial effect on microalgae. In this study, we found that AO significantly increased the expression level of cyclin A, cyclin B, and cyclin D in C. reinhardtii. To our knowledge, this is the first report that AO can influence gene expression in microalga, and the increase in the expression of these cell-cycle related genes may be responsible for the growth promotion. Cyclins are cell cycle regulatory proteins, and stimulate cyclin-dependent kinases (CDKs). Cyclin D, A, and B specifically function at gaps (G1) phase, DNA replication (S) phase, and G2/mitotic (M) phase, respectively.Citation25,26) Since these genes are activated by proliferative stimulus, it is obvious that AO can act as an external stimulant to promote cell division of C. reinhardtii through activation of gene expression. Further studies on intracellular signal transduction leading to activation of clyclin system may provide tips on action mechanism of AO in C. reinhardtii.
There have been several reports on oligosaccharides derived from plant cell walls or microbes that exhibit various bioactivities on plant cells.Citation27) It has been suggested that uronic acid residues in such bioactive oligosaccharides might play an important role in the initiation of certain signal-transduction pathways in plant cells. Furthermore, it has been proposed that uronide-Ca2+ complexes might be the active molecular species that initiate the signal transduction pathways.Citation28)
Previous study found that AO changed the fatty acid composition of C. reinhardtii.Citation20) Especially, the levels of C18:3 n-3 (alpha-linoleic acid), C16:0, and C18:2 cis were significantly increased in C. reinhardtii cultured with AO. In addition, the levels of several fatty acids such as C14:1, C17:0, and C20:0 increased upon the addition of AO even though they were undetectable in C. reinhardtii cultured without AO. Alpha-linoleic acid is involved in the synthesis of jasmonic acid, which regulates plant growth and development as well as plant responses to various stresses.Citation29) Therefore, AO may promote the growth of C. reinhardtii through the increase in alpha-linoleic acid level or modulation of other lipid metabolisms. Further studies on the effect of AO on the lipid metabolism of microalgae linked with lipid-related intracellular signal transduction or gene expression may provide insight into action mechanism of AO.
Heavy metals are known to cause harmful effects on various microalgae.Citation30) In aquaculture firms, CuSO4 is often used for extermination of parasites attached on cultured fish. This metal is known to be toxic to microalga. In fact, our previous study found that CuSO4 showed potent toxic effect on N. oculata, and the addition of 2.5 mg/mL of CuSO4 to the medium resulted in the complete inhibition of the growth of N. oculata.Citation19) Interestingly, the toxic effect of CuSO4 on N. oculata almost completely disappeared by the addition of AO (10 mg/mL).Citation19) Similar to these findings, AO also showed protective effect on CuSO4-induced toxicity in C. reinhardtii. Although the toxic mechanism of CuSO4 on microalgae is not fully understood, oxidative stress induced by CuSO4 may be involved. In fact, analysis using ROS-specific fluorescence probe suggested that intracellular ROS level in C. reinhardtii increased in the presence of CuSO4, and expression levels of antioxidant enzymes such as MSD3 and APX1 in C. reinhardtii cultured with CuSO4 increased, which may reflect a defense system against CuSO4-induced oxidative stress. Addition of AO resulted in the reduction of CuSO4-induced increase in the intracellular ROS level, and decrease in the expression of the antioxidant enzymes. Although the exact action mechanism of AO in CuSO4-induced oxidative stress in C. reinhardtii is still unclear, one can speculate that lowering of intracellular ROS level by AO may lead to partial cancelation of such stress-responsible enzyme-expression.
Since AO showed no chelating activity on metal ion such as Fe2+,Citation31,32) it seems unlikely that AO can act as a chelater for Cu2+ as well. Furthermore, the protective effect of AO against CuSO4 toxicity was the most effective at 2.5 mg/mL but the effect decreased at higher than that concentration. The presence of the optimum concentration may also deny the possibility that the protective effect of AO is due to chelating action against Cu2+.
Since scavenging activities of alginate and AO against ROS have been reported,Citation32,33) there is a possibility that AO directly scavenge intracellular ROS in C. reinhardtii cells. As observed in this study, the effects of AO on C. reinhardtii were not merely concentration-dependent, and the maximum effects of AO on the growth, protective effect against copper ion toxicity, and reduction of intracellular ROS level were attained at 2.5 mg/mL. At higher concentration, the activities rather declined. Hence, there seems to be an optimum concentration of AO to exhibit the maximum beneficial effects on C. reinhardtii. In our previous studies, it was found that AO at 0.125 mg/mL promoted the growth of C. gracilis, but the growth was strongly suppressed at 1 mg/mL, In the case of Skeletonema sp., the growth was significantly inhibited by AO in a concentration-dependent manner at concentration rage of 0–1 mg/mL.Citation22) Although the exact reason is still unclear, the optimum concentration of AO may differ depending on the species, and even the negative effects such as cytotoxicity might be induced when the critical point was exceeded. It is also suggested that simple stoichiometric action mechanism such as chelating against Cu2+ may not be applicable for AO action at least the effects on C. reinhardtii observed in this study. In our preliminary study, it was found that AO level in culture medium of C. reinhardtii was not changed during 4 days incubation, suggesting that C. reinhardtii may not assimilate AO as a carbon source (data not shown).
Recent studies found that alginate hydrolysates showed growth promoting effects on juvenile Manila clam, Ruditapes philippinarum,Citation34) and it is proposed that alginate hydrolysates are taken up by the clam through incurrent siphon together with feeding diatom and absorbed from the alimentary canal.Citation35) These findings together with our results suggest that AO may be multifunctional agent having wide variety of bioactivities toward various organisms, although the action mechanism might differ depending on organisms. Hence, AO can be a promising agent capable of improving growth conditions of variable other microalgae.
Authors contribution
MU conceived and performed the experiments, and analyzed the data together with TN. ST and KY provided valuable advice. TO contributed to the manuscript writing. All authors discussed the results and commented on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported in part by a Grant-in-Aid [15K07580] for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a fund from Nagasaki University Major Research Project (Research Initiative for Adaptation to Future Ocean Change); Grant-in-Aid [14J00356] for Japan Society for the Promotion of Science (JSPS) Fellow to M. Ueno.
Notes
Abbreviations: AO, alginate oligomer; ROS, reactive oxygen species; TNF, tumor necrosis factor; CM-H2DCFDA; carboxy-methyl-2′,7′-dichlorodihydrofluorescein diacetate, AGPC, acid guanidinium-phenol-chloroform; PCR, polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
References
- Haug A. Isolation and fractionation with potassium chloride and manganous ions. Methods Carbohydr Chem. 1965;5:69–73.
- Otterlei M, Østgaard K, Skjåk-Bræk G, et al. Induction of cytokine production from human monocytes stimulated with alginate. J Immunother. 1991;10:286–291.10.1097/00002371-199108000-00007
- Fujihara M, Iizima N, Yamamoto I, et al. Purification and chemical and physical characterization of an antitumor polysaccharide from the brown sea seed Sargassum fulvellum. Carbohydr Res. 1992;125:97–106.
- de Sousa APA, Torres MR, Pessoa C, et al. In vivo growth-inhibition of Sarcoma 180 tumor by alginate from brown seaweed Sargassum vulgare. Carbohydr Polym. 2007;69:7–13.10.1016/j.carbpol.2006.08.018
- Kimura Y, Watanabe K, Okuda H. Effects of soluble sodium alginate on cholesterol excretion and glucose tolerance in rats. J Ethnopharmacol. 1996;54:47–54.10.1016/0378-8741(96)01449-3
- Logeart D, PrigentRicard S, Jozefonvicz J, et al. Fucans, sulfated polysaccharides extracted from brown seaweeds, inhibit vascular smooth muscle cell proliferation. I. Comparison with heparin for antiproliferative activity, binding and internalization. Eur J Cell Biol. 1997;74:376–384.
- Logeart D, PrigentRichard S, BoissonVidal C, et al. Fucans, sulfated polysaccharides extracted from brown seaweeds, inhibit vascular smooth muscle cell proliferation. II. Degradation and molecular weight effect. Eur J Cell Biol. 1997;74:385–390.
- Asada M, Sugie M, Inoue M, et al. Inhibitory effect of alginic acids on hyaluronidase and on histamine release from mast cells. Biosci Biotehnol Biochem. 1997;61:1030–1032.10.1271/bbb.61.1030
- Tajima S, Inoue H, Kawada A, et al. Alginate oligosaccharides modulate cell morphology, cell proliferation and collagen expression in human skin fibroblasts in vitro. Arch Dermatol Res. 1999;291:432–436.10.1007/s004030050434
- Kawada A, Hiura N, Tajima S, et al. Alginate oligosaccharides stimulate VEGF-mediated growth and migration of human endothelial cells. Arch Dermatol Res. 1999;291:542–547.10.1007/s004030050451
- Kawada A, Hiura N, Shiraiwa M, et al. Stimulation of human keratinocyte growth by alginate oligosaccharides, a possible co-factor for epidermal growth factor in cell culture. FEBS Lett. 1997;408:43–46.10.1016/S0014-5793(97)00386-4
- Kurachi M, Nakashima T, Miyajima C, et al. Comparison of the activities of various alginates to induce TNF-α secretion in RAW264.7 cells. J Infect Chemother. 2005;11:199–203.10.1007/s10156-005-0392-0
- Ueno M, Cho L, Nakazono S, et al. Alginate oligomer induces nitric oxide (NO) production in RAW264.7 cells: elucidation of the underlying intracellular signaling mechanism. Biosci Biotechnol Biochem. 2015;79:1787–1793.10.1080/09168451.2015.1052768
- Nakazono S, Cho K, Isaka S, et al. Anti-obesity effects of enzymatically-digested alginate oligomer in mice model fed a high-fat-diet. Bioact Carbohydr Diet Fibre. 2016;7:1–8.10.1016/j.bcdf.2016.02.001
- Yonemoto Y, Tanaka H, Yamashita T, et al. Promotion of germination and shoot elongation of some plants by alginate oligomers prepared with bacterial alginate lyase. J Ferment Bioeng. 1993;75:68–70.10.1016/0922-338X(93)90181-7
- Tomoda Y, Umemura K, Adachi T. Promotion of barley root elongation under hypoxic conditions by alginate lyase-lysate (A.L.L.). Biosci Biotechnol Biochem. 1994;58:202–203.10.1271/bbb.58.202
- Banerjee A, Sharma R, Shisti Y. Botryococcus braunii: a renewable source of hydrocarbons and other chemicals. Crit Rev Biotechnol. 2002;22:245–279.10.1080/07388550290789513
- Kaya K, Nakazawa A, Matsuura H, et al. Thraustochtrid Aurantiochytrium sp. 18 W-13a accummulates high amounts of squalene. Biosci Biotechnol Biochem. 2011;75:2246–2248.10.1271/bbb.110430
- Yokose T, Nishikawa T, Yamamoto Y. Growth-promoting effect of alginate oligosaccharides on a unicellular marine microalga, Nannochloropsis oculata. Biosci Biotechnol Biochem. 2009;73:450–453.10.1271/bbb.80692
- Yamasaki Y, Yokose T, Nishikawa T, et al. Effects of alginate oligosaccharide mixture on the growth and fatty acid composition of the green alga Chlamydomonas reinhardtii. J Biosci Bioeng. 2012;113:112–116.10.1016/j.jbiosc.2011.09.009
- Harris EH. The Chlamydomonas sourcebook. Introduction to Chlamydomonas and its laboratory use. 2nd ed. Vol. 1. San Diego (CA): Academic Press; 2009.
- Yokose T, Yamasaki Y, Nishikawa T, et al. Effects of alginate oligosaccharides on the growth of various mammalian cell lines, unicellular phytoplankters, and marine bactirea. Jpn J Food Chem Safety (in Japanese). 2010;17:27–35.
- Gorman DS, Levine RP. Cytochrome and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Nat Acad Sci USA. 1965;54:1665–1669.
- Tanaka S, Ikeda K, Miyasaka H, et al. Comparison of three Chalamydomonas strains which show distinctive oxidative stress tolerance. J Biosci Bioeng. 2011;112:462–468.10.1016/j.jbiosc.2011.07.019
- Pokora W, Aksmann A, Baścik-Remisiewicz A, et al. Changes in nitric oxide/hydrogen peroxide content and cell cycle progression: Study with synchronized cultures of green alga Chlamydomonas reinhardtii. J Plant Physiol. 2017;208:84–93.10.1016/j.jplph.2016.10.008
- Pestell RG, Albanese C, Reutens AT, et al. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev. 1999;20:501–534.
- Albersheim P, Darvill AG. Oligosaccharides. Sci Am. 1985;253:44–55.
- Farmer EE, Moloshok TD, Saxton MJ, et al. Oligosaccharide signaling in plants specificity of oligouronide-enhanced plasma membrane protein phosphorylation. J Biol Chem. 1991;266:3140–3145.
- Delker C, Stenzel I, Hause B, et al. Jasmonate biosynthesis in arabidopsis thaliana – enzymes, products, regulation. Plant Biol. 2006;8:297–306.10.1055/s-2006-923935
- Satoh A, Vudikaria LQ, Kurano N, et al. Evaluation of the sensitivity of marine microalgal strains to the heavy metals, Cu, As, Sb, Pb and Cd. Environ Int. 2005;31:713–722.10.1016/j.envint.2005.01.001
- Wang P, Jiang X, Jiang Y, et al. In vitro antioxidative activities of three marine oligosaccharides. Nat Prod Res. 2007;21:646–654.10.1080/14786410701371215
- Ueno M, Hiroki T, Takeshita S, et al. Comparative study on antioxidative and macrophage-stimulating activities of polyguluronic acid (PG) and polymannuronic acid (PM) prepared from alginate. Carbohydr Res. 2012;352:88–93.
- Falkeborg M, Cheong LZ, Gianfico C, et al. Alginate oligosaccharides: enzymatic preparation and antioxidant property evaluation. Food Chem. 2014;164:185–194.10.1016/j.foodchem.2014.05.053
- Yamasaki Y, Taga S, Kishioka M. Preliminary observation of growth-promoting effects of alginate hydrolysates on juvenile Manila clams, Ruditapes philippinarum. Aquac Res. 2015;46:1013–1017.10.1111/are.12246
- Yamasaki Y, Taga S, Kishioka M, et al. A metabolic profile in Ruditapes philippinarum associated with growth-promoting effects of alginate hydrolysates. Sci Rep. 2016;6:29923.10.1038/srep29923
- Allen MD, Krooat J, Tottey S, et al. Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function, sensitivity to peroxides, and secondary phosphorus and iron deficiency. Plant Physiol. 2007;143:263–277.
