Abstract
We evaluated the effects of difructose anhydride III (DFAIII) on body weights of ovariectomized rats, which are a good model for obesity by estrogen deficiency-induced overeating. Female rats (10 weeks old) were subjected to ovariectomy or sham operation and then fed with or without a diet containing 3% or 6% DFAIII for 33 days or pair-fed control diet during the same period. Rats fed DFAIII showed significantly decreased food intake, energy intake, body weight gain, body energy accumulation, and fat tissue weight than control group, regardless of ovariectomy. DFAIII may decrease body fat dependent of reduced food/energy intake. Compared with the respective pair feeding groups, rats fed DFAIII showed significantly decreased body energy and fat tissue weight, regardless of ovariectomy, suggesting its potential as a low-energy substitute for high-energy sweeteners. The low energy of DFAIII may contribute to decreased body fat, which may not be dependent on obesity.
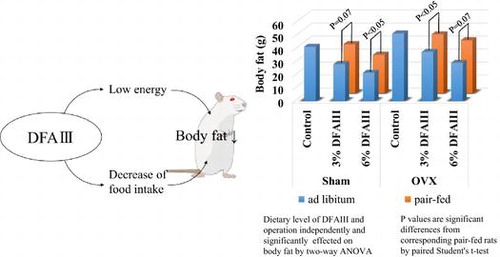
Difructose anhydride III (DFAIII) is a nondigestible disaccharide derived from the roots of Lycoris radiate,Citation1) caramels,Citation2,3) and roasted chicory.Citation4) DFAIII is manufactured from inulin by microbial fermentation.Citation5) Enhancement of intestinal mineral absorption by DFAIII has been reported in rat studies,Citation6,7) and organic acids in the cecum increase substantially with feeding of DFAIII in rats.Citation7) Nondigestible or nonabsorbable saccharides may provide energy by conversion to available organic acids, such as acetic, propionic, and butyric acids. The caloric value of most of nondigestible oligosaccharides has been reported between 1 and 2 kcal/g, which suggests that nondigestible oligosaccharides are fermentable, and resulting organic acids are absorbed from the large intestine and used as an energy source by host animals.Citation8) However, we evaluated body energy accumulation as fat and protein from ingestion of DFAIII and found that the estimated available energy value of DFAIII was 0.263 kcal/gCitation9); this value is 1/15 that of sucrose and one of the lowest reported for oligosaccharides to date.
Some oligosaccharides decrease body fat as a result of decreased food intake. For example, Parnell et al. reported that fructo-oligosaccharide supplementation has the potential to promote weight loss with a reduction in self-reported caloric intake in overweight adults.Citation10) Huang reported that chitosan oligosaccharides decrease body fat in mice fed a high-fat diet, accompanied by decreased food intake.Citation11) In addition, Shinoki et al. reported that fructo-oligosaccharides decrease fat tissue weight without decreasing food intake.Citation12) Thus, oligosaccharides may decrease fat dependent or independent of food intake.
In women, menopause-induced estrogen deficiency and increased androgenicity are associated with increased abdominal obesity and concomitant alterations in the metabolic risk profile.Citation13) Ovariectomized female rats are a good model for obesity caused by estrogen deficiency induced overeating.Citation14)
Accordingly, in the present study, we examine the feasibility of DFA III as low-energy substitute of sucrose in vivo using body energy accumulation as a marker with the pair-feeding method and also examine the effect of DFA III on food intake/energy intake and body fat and against ovariectomy-induced obesity in ad libitum feeding, in non-ovariectomized and ovariectomized female rats.
Materials and methods
Animals
Female Sprague-Dawley rats (Japan SLC, Hamamatsu, Japan; 10 weeks old) were raised in stainless wire mesh cages in a room controlled by a 12-h light/dark cycle (dark phase: 15:00–3:00) and constant temperature (23 ± 1 °C). Rats were housed separately for 3 days to acclimate to the environment. Animals were fed regular tap water and regular chow (trade name: MF; Oriental Yeast, Tokyo, Japan) ad libitum. After acclimation, the rats were divided into two groups of 30 rats each. Under sodium pentobarbital (Nembutal; Dainippon Pharmaceutical, Osaka, Japan; 30 mg/kg weight, intraperitoneal injection) anesthesia, bilateral ovariectomy (OVX) was performed on the first group of rats (OVX-rats), and a sham operation was performed on the second group of rats (sham rats). After a 2-day recovery, during which rats were fed regular chow, the OVX-rats and sham rats were each divided into five groups of six rats. Rats were given free access to the control diet or a diet containing 3% or 6% DFAIII or were pair-fed for 33 days. To examine the feasibility of DFA III as low-energy substitute of sucrose, pair-feeding was performed as follows: a rat in the pair-fed group was paired with a rat having a similar body weight in the DFAIII-fed group at the beginning of the experimental period; the pair-fed rat was fed an amount of the control diet equal to the amount of diet consumed by the paired DFAIII-fed rat the previous day during the experimental period. The control diet contained 200 g/kg casein (New Zealand Dairy Board, Wellington, New Zealand) 50 g/kg cellulose (Danisco Japan, Inc., Tokyo, Japan), 70 g/kg soybean oil (J-oil Mills, Inc., Tokyo, Japan), 35 g/kg AIN-93 mineral mixture, 10 g/kg AIN-93 vitamin mixture, 3 g/kg l-cysteine (Nacalai Tesque, Kyoto, Japan), 100 g/kg sucrose (Nippon Beet Sugar Manufacturing, Tokyo, Japan), and 532 g/kg α-corn starch (Nihon Shokuhin Kako, Tokyo, Japan). The 3% or 6% DFAIII-containing diets included DFAIII at a ratio of 30 or 60 g/kg diet, respectively, replacing sucrose. Energy values of each diet were calculated using Atwater factors (4 kcal/g protein, 4 kcal/g carbohydrate, 9 kcal/g fat) and the energy value of DFAIII (0.263 kcal/g),Citation9) as follows: control diet, 3.63 kcal/g; 3% DFAIII-containing diet, 3.52 kcal/g; and 6% DFAIII-containing diet, 3.40 kcal/g. Body weight and food intake per weight were recorded daily for each rat in the morning before replenishing the diet. After the experimental period, rats were sacrificed by cervical dislocation, and blood samples were collected by decapitation from 13:00 to 15:00. The blood was stored at room temperature for at least 30 min before separating the serum by centrifugation at 3000× g at 4 °C for 15 min and then stored at –50 °C until analysis. The perirenal and ovary fat tissues were removed and weighed; the sum of the weights of the right and left tissues was regarded as the total tissue weight. The carcass was stored at –20 °C until analysis of body composition. This study was conducted in accordance with the ethical guidelines of the Ehime University Animal Experimentation Committee and was in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and limit experimentation to what was necessary to produce reliable scientific information.
Body composition measurement
The carcass was minced. Total body water amount was determined by the difference in the mince weight before and after drying at 105 °C. Total body lipid amount was determined gravimetrically after extraction using the method of Folch et al.Citation15) Total body protein amount was determined using the Kjeldahl method with an N-to-protein conversion factor of 6.25.Citation16) For the determination of glycogen, 1–2 g of the carcass was extracted with 5 mL of 1 M NaOH, incubated for 60 min at 100 °C, treated with10 mL of 1 M HClO4, and brought to a volume of 20 mL with distilled water. After 15 min, the extract was filtered by qualitative filter paper no. 2. Seven milliliters of the filtrate was added to 14 mL of 0.1% LiCl/95% ethanol solution, capped, and incubated overnight at 4 °C. The precipitate was collected by centrifugation (1300× g, 5 min), resuspended in 0.5 mL distilled water, and evaluated colorimetrically (620 nm) using the anthrone/sulfuric acid method.Citation17)
Measurement of serum insulin and glucose levels
Insulin and glucose levels in serum samples were measured using commercial kits, i.e., a rat insulin measurement kit (Morinaga Institute of Biological Science, Inc., Tokyo, Japan) and a glucose assay kit (Wako Pure Chemical Industries, Ltd.), respectively.
Statistical analysis
Total body energy was calculated as aggregation of energy contained in body fat, protein, and glycogen. Body fat was assumed to contain 9.0 kcal/g, and body protein and glycogen were assumed to contain 4.0 kcal/g. Data are expressed as the mean ± standard error (SEM). Data from weekly food intake were analyzed by two-way repeated measures analysis of variance (ANOVA) with Bonferroni post hoc significance testing. Other data were analyzed by two-way ANOVA with Bonferroni post hoc significance testing. The comparisons between groups fed 3% or 6% DFAIII and the respective pair-fed group were carried out using paired Student’s t-tests. Statistical significance was defined as p < 0.05. All statistical tests were done with IBM SPSS Statistics software (SPSS Japan Inc., an IBM company).
Results
Food intake, energy intake, body weight, and body weight gain
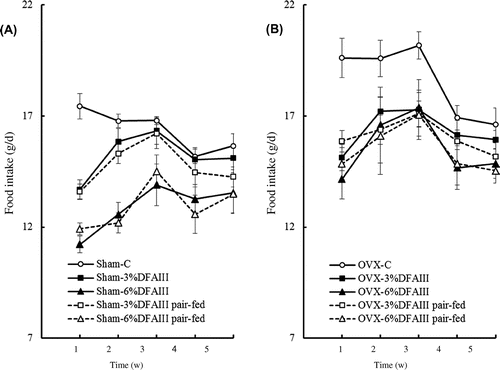
OVX significantly increased food intake, energy intake, final body weight, and body weight gain during the 33-day test period. Dietary DFAIII significantly reduced these parameters, and there were no interaction between OVX and dietary levels of DFAIII. The weekly food intake in rats fed a diet containing 6% DFAIII was significantly lower than that in rats fed the control diet from week 1 to week 5, and the weekly food intake in rats fed a diet containing 3% DFAIII was significantly lower than that in rats fed the control diet in week 1, regardless of whether the rats had undergone OVX; a significant interaction between dietary levels of DFAIII and time was observed in the weekly food intake. Food intake was well matched between paired groups, regardless of whether the rats had undergone OVX. The energy intake and final body weight in rats fed a diet containing 6% DFAIII was significantly lower than that in the corresponding pair-fed group, regardless of whether the rats had undergone OVX. The body weight gain in rats fed 6% DFAIII was significantly lower than that in the corresponding pair-fed group in OVX rats. During the experimental period, one of the four rats in the group fed 3% DFAIII and none of the rats in the group fed 6% DFAIII showed minor diarrhea (see Table and Fig. ).
Table 1. Food intake, energy intake, body weight, and body weight gain.Table Footnotea
Fig. 1. Weekly changes in food intake in sham female rats (A) and ovariectomized (OVX) rats (B) fed the control diet (open circle), fed a diet containing 3% DFAIII (closed square), fed a diet containing 6% DFAIII (closed triangle), pair-fed with 3% DFAIII (open square), or pair-fed with 6% DFAIII (open triangle) for 5 weeks.
Body energy accumulation, body fat, protein content, water content, and glycogen content
OVX significantly increased body energy accumulation and body fat. Dietary DFAIII significantly decreased body energy accumulation, body fat, and body glycogen, and there were no interaction between OVX and dietary levels of DFAIII. The body energy in rats fed 6% DFAIII was significantly lower than that in the corresponding pair-fed group only in sham rats, while there were no significant differences between other paired groups, regardless of whether the rats had undergone OVX. Body fat in rats fed 6% DFAIII was significantly lower than that in the corresponding pair-fed group in sham rats, and body fat in rats fed 3% DFAIII was significantly lower than that in the corresponding pair-fed group in OVX rats; the same trends were observed between other paired groups, regardless of whether the rats had undergone OVX. In contrast to the results for body fat, body glycogen in rats-fed DFAIII was significantly higher than that in the respective pair-fed groups, regardless of whether the rats had undergone OVX (see Table ).
Table 2. Body energy, body fat, protein content, water content, and glycogen content.Table Footnotea
Fat tissue weight
OVX significantly increased perirenal fat pad weight. Dietary DFAIII significantly decreased perirenal and ovary fat pad weights, and there were no interaction between OVX and dietary levels of DFAIII. The perirenal fat pad weight in rats fed a diet containing 3% DFAIII was significantly lower than that in the corresponding pair-fed groups in both the sham rats and the OVX rats, the same trend was observed in rats fed a diet containing 6% DFAIII, although there was a significant difference in only the OVX rats. The ovary fat pad weight in rats fed a diet containing 3% DFAIII was significantly lower than that in the corresponding pair-fed groups in both the sham rats and the OVX rats, the same trend was observed in rats fed a diet containing 6% DFAIII, although there was a significant difference in only the sham rats (see Table ).
Table 3. Fat tissue weights.Table Footnotea
Serum concentrations of glucose and insulin
OVX significantly decreased serum glucose concentration. Dietary DFAIII also decreased serum glucose concentration, and there was no interaction between OVX and dietary levels of DFAIII. Rats fed the DFAIII diet showed significantly increased serum glucose concentration relative to that in corresponding pair-fed rats. Serum glucose concentration in rats fed a diet containing 3% DFAIII was significantly lower than that in the corresponding pair-fed groups in both the sham rats and the OVX rats, and serum glucose concentration in rats fed a diet containing 6% DFAIII was significantly lower than that in the corresponding pair-fed groups in only the sham rats. There were no significant differences in serum insulin concentration (see Table ).
Table 4. Serum concentrations of glucose and insulin.Table Footnotea
Discussion
In our previous study, we estimated available energy of DFAIII at 0.263 kcal/g, which was much lower than the estimated available energy of other sugars.Citation9) DFAIII has half of the sweetness of sucrose, and the quality of sweetness is similar to that of fructose.Citation18) In the present study, dietary DFAIII significantly decreased the energy intake, body energy accumulation and body fat in a dose-dependent manner, regardless of whether the rats had undergone OVX. In addition, the body fat in the rats fed 6% DFAIII was significantly lower than that in pair-fed rats in the sham-rats, that in the rats fed 3% DFAIII were significantly lower than that in the pair-fed rats in the OVX rats, and same trend was observed between other paired groups, regardless of whether the rats had undergone OVX. Thus, the low energy of DFAIII may contribute to decreased body fat, demonstrating the feasibility of DFAIII as a low-energy substitute for high-energy sweeteners, such as sucrose.
In the present study, for rats fed ad libitum, the decrease in energy intake resulting from dietary DFAIII caused decreased body energy, body fat, and adipose tissue weight. Similar to our results, previous studies have shown that some oligosaccharides decrease body fat as a result of decreased food intake. For example, chitosan oligosaccharide decreases body fat in mice fed a high-fat diet, accompanied by decreased food intake.Citation11) Although we did not examine mechanistic parameters in this study, wheat-derived arabinoxylan oligosaccharide has been shown to decrease body fat in high-fat diet-induced obese mice by increasing satiety hormones, peptide YY, and glucagon like peptide-1, without significant decreases in food intake.Citation19) Additional studies, including evaluation of appetite-regulating hormones, are needed.
In the present study, slight diarrhea was observed only in rats fed 6% DFAIII. Diarrhea may affect food intake and nutrient absorption, and the decreases in body fat and adipose tissue in this group may be partially explained by diarrhea. However, these parameters were also significantly decreased in rats fed 3% DFAIII, without the occurrence of diarrhea. Thus, we speculate that the effects of diarrhea may be limited.
In the present study, we evaluated the body glycogen content to precisely measure body energy. Body glycogen in rats fed 3% or 6% DFAIII was significantly higher than that in corresponding pair-fed groups, despite the lower-energy intake in the rats fed ad libitum with a diet containing 3% or 6% DFAIII. Notably, however, the energy in body glycogen was too small to significantly affect whole-body energy. Matsuo et al. reported that the low-calorie monosaccharide psicose increases rat liver glycogen content both before and after meals relative to sucrose and fructose feeding.Citation20) Bar et al. also reported the same phenomenon in tagatose feeding.Citation21) Increased liver glycogen may contribute to the increase in body glycogen following consumption of dietary DFAIII, although in the present study we only evaluated whole-body glycogen. In addition, it has been reported that the glycogen stores in liver, skeletal muscle, heart and adipose tissue show diurnal changes and the glycogen peaks in liver and skeletal muscle could be obtained about 8 hours after the sucrose ingestion in rats.Citation22) Food restriction may alter the timing of meal ingestion and glycogen peaks in pair-fed rats.
In this study, although an increase in body fat caused by estrogen deficiency-induced overeating was observed in OVX rats, rats fed the DFAIII diet showed significantly decreased body fat, regardless of whether rats had undergone OVX, relative to rats fed the control diet. The effects of DFAIII on body fat expressed equally in both sham female rats and OVX rats, which may not be dependent on obesity. However, in the present study, body protein content was not decreased following consumption of 3% or 6% DFAIII, and the minor diarrhea observed was not expected to have effects on body protein content.
In conclusion, we found that the low energy of DFAIII contributed to decreased body fat when used as a substitute for sucrose, demonstrating the feasibility of DFAIII as a low-energy substitute for high-energy sweeteners. In addition, we found that DFAIII also decreased body fat dependent or independent of decreased food/energy intake, suggesting its feasibility as a functional food.
Author contribution
M.F. conducted most of the experiments, analyzed the results with the aids by E.S., and wrote most of the paper. T.K. contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript. J.I. contributed to conception and design of the study. All the authors reviewed the results and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Li HY, Hagiwara H, Zhu W, et al. Isolation and NMR studies of di-d-fructose anhydride III from Lycoris radiata Herbert by supercritical extraction with carbon dioxide. Carbohydrate Research. 1997;299:301–305.10.1016/S0008-6215(97)00016-5
- Manley-Harris M, Richards GN. Di-d-fructose dianhydrides and related oligomers from thermal treatments of inulin and sucrose. Carbohyd Res. 1996;287:183–202.10.1016/0008-6215(96)00071-7
- Ratsimba V, Garcia-Fernandez JM, Defaye J, et al. Qualitative and quantitative evaluation of mono- and disaccharides in d-fructose, d-glucose and sucrose caramels by gas–liquid chromatography–mass spectrometry. J Chromatgr A. 1999;844:283–293.10.1016/S0021-9673(99)00322-2
- Defaye J, Garcia-Fernandez JM. The oligosaccharide components of caramels. Zuckerind. 1995;120:700–704.
- Saito K, Tomita F. Difructose anhydrides: their mass-production and physiological functions. Biosci Biotechnol Biochem. 2000;64:1321–1327.10.1271/bbb.64.1321
- Suzuki T, Hara H, Kasai T, et al. Effects of difructose anhydride III on calcium absorption in small and large intestines of rats. Biosci Biotechnol Biochem. 1998;62:837–841.10.1271/bbb.62.837
- Mitamura R, Hara H, Aoyama Y, et al. Supplemental feeding of difructose anhydride III restores calcium absorption inpaired by ovariectomy in rats. J Nutr. 2002;132:3387–3393.
- Livesey G. The energy values of dietary fibre and sugar alcohols for man. Nutr Res Rev. 1992;5:61–84.10.1079/NRR19920007
- Tamura A, Nino H, Minobe T, et al. Difructose anhydride III does not contribute to body energy accumulation in rats. Biosci Biotechnol Biochem. 2006;70:1416–1422.10.1271/bbb.50666
- Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89:1751–1759.10.3945/ajcn.2009.27465
- Huang L, Chen J, Cao P, et al. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Marine Drugs. 2015;13:2732–2756.10.3390/md13052732
- Shinoki A, Hara H. Dietary fructo-oligosaccharides improve insulin sensitivity along with the suppression of adipocytokine secretion from mesenteric fat cells in rats. Br J Nutr. 2011;106:1190–1197.10.1017/S000711451100167X
- Tchernof A, Després JP. Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm Metab Res. 2000;32:526–536.10.1055/s-2007-978681
- Kishida T, Mizushige T, Ohtsu Y, et al. Dietary soy isoflavone–aglycone lowers food intake in female rats with and without ovariectomy. Obesity. 2008;16:290–297.10.1038/oby.2007.68
- Folch J, Less M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem. 1957;226:497–509.
- Miller L, Houghton JA. The micro-Kjeldahl determination of the nitrogen content of amino acids and proteins. J Biol Chem. 1945;159:373–380.
- Roe JH. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1954;208:889–896.
- Kikuchi H, Nagura T, Inoue M, et al. Physical, chemical and physiological properties of difructose anhydride III produced from inulin by enzymatic reaction. J Appl Glycosci. 2004;51:291–296.10.5458/jag.51.291
- Neyrinck AM, Van Hée VF, Piront N, et al. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes. 2012;2:e28.10.1038/nutd.2011.24
- Matsuo T, Izumori K. Effects of dietary D -psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci Biotechnol Biochem. 2006;70:2081–2085.10.1271/bbb.60036
- Bär A, Lina BA, de Groot DM, et al. Effect ofd-tagatose on liver weight and glycogen content of rats. Regulatory Toxicology and Pharmacology. 1999;29:S11–S28.10.1006/rtph.1998.1266
- Suzuki M, Ide K, Saltoh S. Diurnal changes in glycogen stores in liver and skeletal muscle of rats in relation to the feed timing of sucrose. J Nutr Sci Vitaminol (Tokyo). 1983;29(5):545–552.10.3177/jnsv.29.545
