Abstract
In Euglena gracilis, pyruvate:NADP+ oxidoreductase, in addition to the pyruvate dehydrogenase complex, functions for the oxidative decarboxylation of pyruvate in the mitochondria. Furthermore, the 2-oxoglutarate dehydrogenase complex is absent, and instead 2-oxoglutarate decarboxylase is found in the mitochondria. To elucidate the central carbon and energy metabolisms in Euglena under aerobic and anaerobic conditions, physiological significances of these enzymes involved in 2-oxoacid metabolism were examined by gene silencing experiments. The pyruvate dehydrogenase complex was indispensable for aerobic cell growth in a glucose medium, although its activity was less than 1% of that of pyruvate:NADP+ oxidoreductase. In contrast, pyruvate:NADP+ oxidoreductase was only involved in the anaerobic energy metabolism (wax ester fermentation). Aerobic cell growth was almost completely suppressed when the 2-oxoglutarate decarboxylase gene was silenced, suggesting that the tricarboxylic acid cycle is modified in Euglena and 2-oxoglutarate decarboxylase takes the place of the 2-oxoglutarate dehydrogenase complex in the aerobic respiratory metabolism.
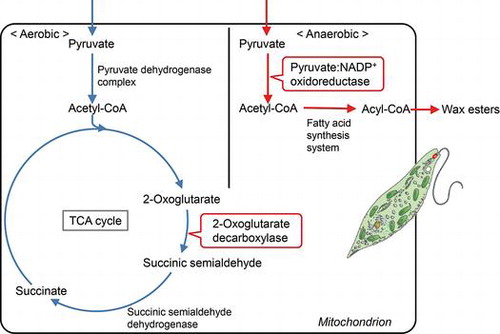
In most eukaryotes, the oxidative decarboxylation of pyruvate and 2-oxoglutarate is catalyzed by a corresponding 2-oxoacid dehydrogenase multienzyme complex.Citation1,2) The multienzyme complex is a large assembly with a molecular mass higher than 1,000 kDa, and consists of three component enzymes, pyruvate or 2-oxoglutarate dehydrogenase (E1), dihydrolipoamide acetyltransferase or succinyltransferase (E2), and dihydrolipoamide dehydrogenase (E3). In the multienzyme complex, the reducing power of the substrate is transferred to NAD+, and acetyl-CoA or succinyl-CoA is formed with CoA as a substrate. In contrast, in archaea, most anaerobic eubacteria and amitochondriate eukaryotes, pyruvate:ferredoxin or 2-oxoglutarate:ferredoxin oxidoreductase functions in a similar reaction using ferredoxin as an electron acceptor with a more negative redox potential than NAD+.Citation3)
Euglena gracilis, a unicellular protist containing chloroplasts, can grow either photoautotrophicallyCitation4) or heterotrophically with certain carbon sources such as glucose or ethanol.Citation5,6) When Euglena cells grown under aerobic conditions are placed in anaerobiosis, paramylon, the storage polysaccharide of this organism, is promptly converted to wax esters consisting of middle-length chain saturated fatty acids and alcohols, and ATP is generated during the conversion (wax ester fermentation).Citation7,8) In wax ester fermentation, fatty acids are synthesized in the mitochondria by the reversal of β-oxidation with trans-2-enoyl-CoA reductase taking the place of acyl-CoA dehydrogenase.Citation9) In this fatty acid synthesis, acetyl-CoA is used both as a C2 donor and a primer, and malonyl-CoA which is synthesized by the ATP-dependent acetyl-CoA carboxylase reaction is not required. Consequently, the mitochondrial fatty acid synthesis system makes possible the net gain of ATP through the synthesis of wax esters from the storage polysaccharide.
In addition to the common pyruvate dehydrogenase multienzyme complex, pyruvate:NADP+ oxidoreductase catalyzes the oxidative decarboxylation of pyruvate in the mitochondria of E. gracilis.Citation10,11) Pyruvate:NADP+ oxidoreductase is a homodimeric protein with a molecular mass of 390 kDa, and each subunit consists of two functional domains.Citation12) One of the domains located on the N-terminal side has three [4Fe-4S] clusters and thiamin pyrophosphate as prosthetic groups, and structurally resembles pyruvate:ferredoxin oxidoreductase found in amitochondriate eukaryotes. The other domain contains FAD and FMN, and is structurally similar to certain flavoproteins such as NADPH-cytochrome P450 reductase. Pyruvate:NADP+ oxidoreductase, like pyruvate:ferredoxin oxidoreductase, is oxygen-labile in vitro,Citation13) and is thought to play a pivotal role in the wax ester fermentation.Citation14) On the other hand, the enzyme seems to be stable in vivo even under aerobic conditions, because a relatively high activity is detected in the mitochondria of aerobically grown cells in air.Citation13) However, it is unclear whether pyruvate:NADP+ oxidoreductase, together with the pyruvate dehydrogenase complex, participates in the aerobic respiratory metabolism in this organism.
The 2-oxoglutarate dehydrogenase complex is absent in E. gracilis, and instead 2-oxoglutarate decarboxylase is found in the mitochondria.Citation15,16) 2-Oxoglutarate decarboxylase is a thiamin pyrophosphate-dependent enzyme that catalyzes the non-oxidative decarboxylation of 2-oxoglutarate to form succinic semialdehyde. It is thought that the tricarboxylic acid (TCA) cycle is modified in Euglena, and 2-oxoglutarate is converted to succinate by the cooperation of 2-oxoglutarate decarboxylase and succinate semialdehyde dehydrogenase.Citation15,17) However, there is no direct evidence showing that 2-oxoglutarate decarboxylase participates in the aerobic respiratory metabolism. In addition, the evolutional origin of this enzyme in this organism is not known.
In the present paper, to clarify unique metabolic pathways involved in the aerobic respiration and anaerobic wax ester fermentation in E. gracilis, physiological roles of the pyruvate dehydrogenase complex, pyruvate:NADP+ oxidoreductase and 2-oxoglutarate decarboxylase are studied by silencing the genes of these enzymes. We report that the pyruvate dehydrogenase complex and pyruvate:NADP+ oxidoreductase have different physiological functions, and participate in the aerobic respiratory metabolism and in anaerobic wax ester fermentation, respectively. We also show that 2-oxoglutarate decarboxylase is indispensable for the respiratory metabolism to sustain cell growth.
Materials and Methods
Organism and culture
E. gracilis SM-ZK, non-photosynthetic mutant strain,Citation18) was cultured in Koren-Hutner (KH) medium containing glucose as the major carbon sourceCitation19) with aeration at 27 °C for 4 days to the late logarithmic phase of growth. The cells were also cultured in an inorganic mediumCitation4) supplemented with 85.6 mM ethanol (ethanol medium). Anaerobic cells were prepared by transferring an aliquot of 1.5 mL aerobic culture into a 1.5 mL plastic tube, which was then tightly capped and allowed to stand without agitation at 27 °C for 24 h. Cells were counted with a hemocytometer or a particle counter CDA-1000 (Sysmex, Japan). Cell viability was evaluated by propidium iodide staining according to the method described by Nakazawa et al.Citation20)
Purification of 2-oxoglutarate decarboxylase and peptide mapping
2-Oxoglutarate decarboxylase was purified from Euglena cells according to the method described by Shigeoka & Nakano.Citation16) The N-terminal amino acid sequence was determined using the Model 491 Procise Protein Sequencer (Applied Biosystems, MA, USA). To determine internal amino acid sequences, the purified enzyme was partially cleaved by lysylendopeptidase on a polyvinylidene difluoride membrane. Digested peptides were separated using high performance liquid chromatography and applied to the protein sequencer.
Cloning
Partial 2-oxoglutarate decarboxylase cDNA fragments were amplified from Euglena total cDNACitation21) with degenerate primers designed according to the amino acid sequences obtained by peptide mapping: the N-terminal amino acid sequence IALDGPDRMFNAYLH and internal amino acid sequence DLVHVAINTADLIVNVGHDI. An amplified cDNA fragment obtained by PCR (ca 900 bp) was purified and sequenced. Sequential plaque screening of a Euglena cDNA library was carried out using the partial 2-oxoglutarate decarboxylase PCR fragment labeled with alkaline phosphatase as a probe. The longest cDNA clones isolated from the positive plaque were sequenced and analyzed by BLAST on the NCBI databases.
Heterologous expression of Euglena 2-oxoglutarate decarboxylase
Recombinant Euglena 2-oxoglutarate decarboxylase was produced in Escherichia coli. Nucleotide fragments of 2-oxoglutarate decarboxylase cDNA were obtained by PCR using the cloned phagemid from the cDNA library as a template. The 2-oxoglutarate decarboxylase open reading frame without the coding region for an N-terminal signal peptide was subcloned into expression plasmid pMAL-c2X (New England Biolabs, MA, USA) to express the protein as a maltose binding protein (MBP)-fusion protein. The recombinant protein was expressed by inducing with isopropyl thio-β-galactoside at 13 °C for 24 h in E. coli JM109, and purified with affinity chromatography and gel filtration.
RNA interference
Double-strand RNA was synthesized and purified using a MEGAscript RNAi kit (Thermo Fisher Scientific, MA, USA), and total RNA extracted from Euglena cells was reverse transcribed with SuperScript III reverse transcriptase (Thermo Fisher Scientific, MA, USA) according to the method described by Nakazawa et al.Citation20) A template DNA containing T7 RNA polymerase promoters on both ends was amplified from Euglena cDNA by PCR with primers listed in Table .
Table 1. Sequences of oligonucleotide primers used in the study.
Euglena cells cultured in KH or ethanol medium for 4 days were collected, washed in PBS (+) twice, and resuspended in PBS (+). The cell suspension containing 4 × 106 Euglena cells (400 μL) was transferred to a 0.2-cm-gap cuvette and electroporated with 30 μg of dsRNA. In the control experiment, 30 μL of Tris-EDTA (TE) buffer (pH 8.0, containing no dsRNA) was introduced. The conditions of electroporation, using ECM630 (BTX, MA, USA), were 0.5 kV and 200 μF. After electroporation, 1 mL of culture medium was added to the cell suspension; the culture was incubated at room temperature (27 °C) with gentle shaking using a gyratory shaker. After 3 h, aliquots were diluted in KH or ethanol medium and cultured with continuous shaking (120 rpm) at 27 °C. Cells were collected with centrifugation each day after dsRNA introduction. Crude cell extracts were prepared as described previously.Citation10,15)
Determination of enzymatic activities
The activity of pyruvate dehydrogenase complex was assayed with p-iodonitrotetrazolium violet as a final electron acceptor according to the method of Schwab et al.Citation22) Pyruvate:NADP+ oxidoreductase,Citation10) 2-oxoglutarate decarboxylase,Citation15) acetolactate synthase,Citation23) and pyruvate decarboxylaseCitation24) were assayed according to the methods of references cited. Protein concentrations were measured according to BradfordCitation25) using bovine serum albumin as a standard.
Western blotting
The crude cell extract was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% gel) according to Laemmli.Citation26) Proteins in the gel were transferred to a polyvinylidene difluoride membrane (Immobilon-P; Merck Millipore, MA, USA) by electroblotting. The membrane was treated with the antibodies listed below to detect each enzyme: rabbit polyclonal antibody against the peptide HSLAKAHRREGGET (amino acid position 76–89; to detect Euglena 2-oxoglutarate decarboxylase), rabbit polyclonal antibody against the peptide CLERFLDDEETKG (amino acid position 200–212; to detect Euglena succinyl-CoA synthetase alpha subunit), mouse polyclonal antibody against pyruvate:NADP+ oxidoreductase,Citation27) and mouse monoclonal antibody against the pyruvate dehydrogenase complex E1alpha subunit (8D10E6, Abcam, UK). Immunoreactive proteins on the membrane were detected with horseradish peroxidase-conjugated antibodies (New England BioLabs, MA, USA) and EzWestLumi plus (ATTO, Japan) based on a chemiluminescent detection system. Bands were visualized using the LAS-4000 Image reader (GE healthcare, UK).
Determination of wax ester content
Euglena cells after anaerobic treatment were collected by centrifugation (17,500 × g for 1 min). Extraction of lipid fractions containing wax esters from Euglena cells was done according to the method in Bligh and Dyer.Citation28) The lipid fractions were evaporated and dissolved in chloroform. Wax esters were determined by gas chromatography according to Nakazawa et al.Citation20)
Results and discussion
Difference in physiological roles of the pyruvate dehydrogenase complex and pyruvate:NADP+ oxidoreductase
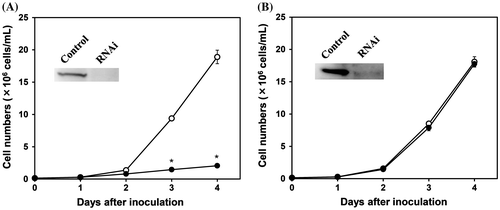
In E. gracilis, pyruvate:NADP+ oxidoreductase, in addition to the common pyruvate dehydrogenase complex, is found as an enzyme that catalyzes the oxidative decarboxylation of pyruvate to form acetyl-CoA in the mitochondria.Citation10,11) The activity of pyruvate:NADP+ oxidoreductase in Euglena cells grown aerobically in KH medium, in which glucose is the major carbon source, was 180 nmol/mg protein/min, which was over 100 times that of the pyruvate dehydrogenase complex (1.3 nmol/mg protein/min). In contrast, pyruvate:NADP+ oxidoreductase is known to be an oxygen-labile enzyme, and is readily inactivated in the presence of oxygen in vitro.Citation13) To determine whether the pyruvate dehydrogenase complex or pyruvate:NADP+ oxidoreductase functions for the oxidative decarboxylation of pyruvate in the mitochondria of aerobically grown Euglena cells, effects of the gene silencing of these enzymes on aerobic cell growth in a glucose medium was examined. Gene silencing of the pyruvate dehydrogenase complex E1alpha, confirmed by Western blotting, caused a marked decrease in the aerobic growth of Euglena in KH medium (Fig. (A)). In contrast, Euglena cells were able to grow normally in KH medium in aerobiosis when the pyruvate:NADP+ oxidoreductase gene was silenced (Fig. (B)). These results indicate that the pyruvate dehydrogenase complex functions to provide acetyl-CoA from pyruvate into the TCA cycle in aerobically grown Euglena cells, although its activity is extremely low compared with pyruvate:NADP+ oxidoreductase. In addition, these results also suggest that the oxidative decarboxylation of pyruvate is not catalyzed by pyruvate:NADP+ oxidoreductase in the aerobically grown cells, even if the multienzyme complex is deleted.
Fig. 1. Effects of gene silencing of the pyruvate dehydrogenase complex and pyruvate:NADP+ oxidoreductase on the aerobic growth of Euglena.
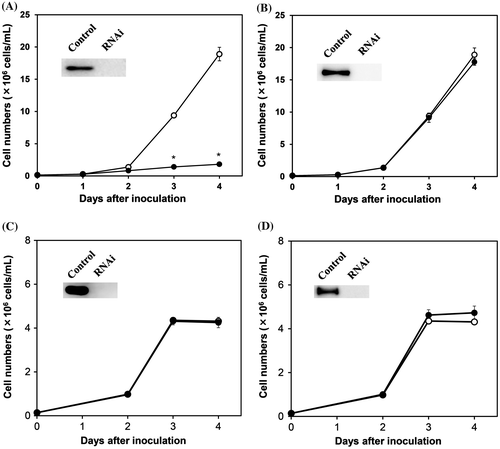
When Euglena cells were cultured in ethanol medium under aerobic conditions, no significant effect on growth was observed in the pyruvate dehydrogenase complex E1alpha gene-silenced cells or in the pyruvate:NADP+ oxidoreductase gene-silenced ones (Fig. (C,D)). Acetyl-CoA would be provided into the TCA cycle directly from ethanol through alcohol and aldehyde dehydrogenase reactions.Citation29,30) It is thus indicated that the pyruvate dehydrogenase complex is required only for converting pyruvate to acetyl-CoA, and Euglena cells, even if the multienzyme complex is deleted, can be cultured aerobically by using ethanol as a carbon source.
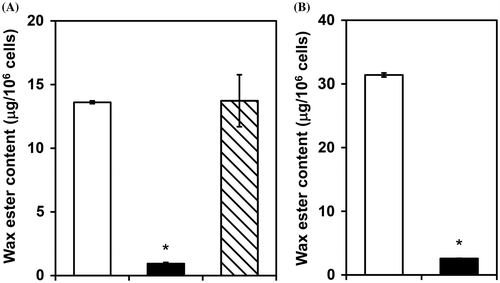
When Euglena cells are exposed to low oxygen conditions, wax ester fermentation is induced and wax esters are promptly produced from the storage polysaccharide, paramylon, to generate ATP.Citation7) As shown in Fig. (A), silencing the pyruvate dehydrogenase complex E1alpha gene did not have any effect on wax ester synthesis after transfer to anaerobic conditions in cells cultured aerobically in ethanol medium. In contrast, wax ester synthesis decreased markedly after transfer to anaerobic conditions when the pyruvate:NADP+ oxidoreductase gene was silenced. A similar result was observed when the pyruvate:NADP+ oxidoreductase gene-silenced cells were cultured aerobically in KH medium and then transferred to anaerobiosis (Fig. (B)). It is thus reasonable to postulate that the pyruvate dehydrogenase complex and pyruvate:NADP+ oxidoreductase have different physiological functions: the former plays an important role in providing acetyl-CoA into the TCA cycle in the aerobic mitochondria, while the latter only functions to provide acetyl-CoA into the mitochondrial fatty acid synthesis system in anaerobic wax ester fermentation.
Fig. 2. Effects of gene silencing of the pyruvate dehydrogenase complex and pyruvate:NADP+ oxidoreductase on anaerobic wax ester fermentation.
When the pyruvate:NADP+ oxidoreductase gene-silenced cells were cultured aerobically in KH medium and then transferred to anaerobiosis, the number of living cells decreased significantly during anaerobic incubation (Table ). We previously observed that few wax esters were produced and the number of living cells decreased when thiamin-deficient Euglena cells with lowered the enzyme activity were exposed to anaerobiosis.Citation31) These results indicate that pyruvate:NADP+ oxidoreductase is important to Euglena for survival under low oxygen conditions.
Table 2. Effect of pyruvate:NADP+ oxidoreductase silencing on cell viability in aerobic and anaerobic conditions.
Characterization of Euglena 2-oxoglutarate decarboxylase
To address the evolutional origin of Euglena 2-oxoglutarate decarboxylase, its cDNA was cloned. The obtained cDNA was 2415 bp in length (the nucleotide sequence data were deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under the accession number AB073207), and had a spliced leader sequence. This sequence is commonly found at the 5′ end of the vast majority of cytoplasmic mRNAs in Euglena.Citation32) The 2-oxoglutarate decarboxylase cDNA encoded a polypeptide composed of 637 amino acid residues. The N-terminal amino acid in the mature form of this enzyme (isoleucine residue) was found at position 22 in the amino acid sequence deduced from the cDNA, suggesting that the precursor form of the enzyme has a 21 amino acid-long peptide as a mitochondrial targeting sequence (underlined in Fig. ). The calculated molecular mass of the mature polypeptide was 67.5 kDa.
Fig. 3. Multiple alignments of the deduced amino acid sequence of Euglena 2-oxoglutarate decarboxylase and its related enzymes.
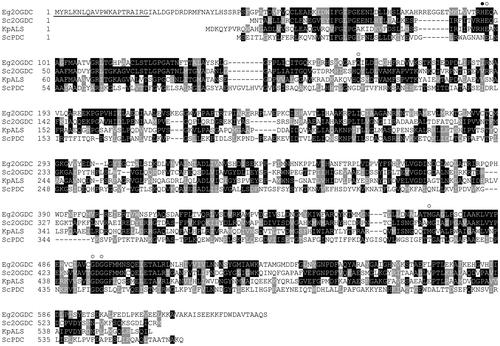
In addition to Euglena, 2-oxoglutarate decarboxylase is also found in Leuconostoc oenos,Citation33) Mycobacterium tuberculosisCitation34) and cyanobacteria.Citation35,36) 2-Oxoglutarate decarboxylase is thought to be divided into at least two types that have evolved from phylogenetically different origins, since no significant homology is observed between the enzymes of Mycobacterium and cyanobacteria.Citation35) The deduced amino acid sequence of Euglena 2-oxoglutarate decarboxylase (Fig. ) resembled that of cyanobacterial 2-oxoglutarate decarboxylase (37 and 38% positional identities, respectively, to the enzymes from Synechococcus sp. PCC 7002Citation35) and Synechocystis sp. PCC 6803Citation36)). In addition, like cyanobacterial 2-oxoglutarate decarboxylase, Euglena 2-oxoglutarate decarboxylase shared a significant homology with acetolactate synthase (30, 24, 25% positional identities, respectively, with the enzymes from Klebsiella pneumonia,Citation37) Saccharomyces cerevisiaeCitation38) and Arabidopsis thalianaCitation39)) and also with pyruvate decarboxylase (22 and 18% positional identities, respectively, with the enzymes from S. cerevisiaeCitation40) and Zymomonas mobilisCitation24)). In contrast, as expected, there was no significant structural similarity between the Euglena and Mycobacterium 2-oxoglutarate decarboxylase.
Euglena 2-oxoglutarate decarboxylase was expressed as an MBP-fusion protein in E. coli, and purified by affinity chromatography and gel filtration. The recombinant protein catalyzed the 2-oxoglutarate decarboxylase reaction dependent on thiamin pyrophosphate; however, neither acetolactate synthase nor pyruvate decarboxylase activity was detected. These results indicate that Euglena 2-oxoglutarate decarboxylase is specific for 2-oxoglutarate, and pyruvate is not used as a substrate.
The physiological functions of 2-oxoglutarate decarboxylase in Euglena
To elucidate the physiological role of 2-oxoglutarate decarboxylase in Euglena, its gene was silenced and the cells were cultured in KH medium in aerobic conditions. As shown in Fig. (A), the growth was almost completely suppressed in the silenced cells. This result indicates that Euglena, which lacks the 2-oxoglutarate dehydrogenase complex, cannot grow under aerobic conditions without 2-oxoglutarate decarboxylase. Succinyl-CoA synthetase, which catalyzes the conversion of succinyl-CoA to succinate, followed by the 2-oxoglutarate dehydrogenase complex in the common TCA cycle, was found in Euglena cells. Hence, to examine whether succinyl-CoA synthetase participates in the respiratory metabolism in aerobic cells, the gene of the succinyl-CoA synthetase alpha subunit was silenced (Fig. (B)). No effect on growth under aerobic conditions was observed.
Fig. 4. Effects of gene silencing of 2-oxoglutarate decarboxylase and succinyl-CoA synthetase on the aerobic growth of Euglena.
These results show that Euglena cells have a modified TCA cycle in the mitochondria, in which 2-oxoglutarate decarboxylase and succinic semialdehyde dehydrogenase function to convert 2-oxoglutarate to succinate instead of the 2-oxoglutarate dehydrogenase complex and succinyl-CoA synthetase. A similar modified TCA cycle is found in cyanobacteria,Citation35,36) where it is reported that the γ-aminobutyrate shunt, in addition to 2-oxoglutarate decarboxylase, participates in the conversion of 2-oxoglutarate to succinic semialdehyde, and growth is slightly reduced when 2-oxoglutarate decarboxylase is deleted.Citation36) Enzymes involved in the γ-aminobutyrate shunt, i.e. glutamate decarboxylase and γ-aminobutyrate:2-oxoglutarate aminotransferase, are also found in Euglena mitochondria.Citation41) However, our present results strongly suggest that in Euglena, in contrast to cyanobacteria, the γ-aminobutyrate shunt has little effect on the metabolism of 2-oxoglutarate, and 2-oxoglutarate decarboxylase is indispensable to produce sufficient ATP in the respiratory metabolism to sustain cell growth.
Euglena is one of the most attractive resources for biofuel production, because many wax esters suitable to convert into a drop-in-jet fuel accumulate under anaerobic conditions.Citation20) Furthermore, nitrogen-starved Euglena cells, when incubated anaerobically in the presence of NaHCO3, excrete succinate that is used as a building block for various compounds including plastic polybutylene succinate.Citation42) Results obtained in the present study on 2-oxoacid metabolism would be useful not only to understand the central carbon and energy metabolisms but also to improve the productivity of wax esters and succinate by gene manipulation in E. gracilis.
Author contributions
All members conceived and designed the experiments, and discussed the results for the completion of the manuscript. M. N. and R. H. performed the experiments and analyzed the data. S. T. performed the peptide sequence analysis. M. N. and H. I. wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research is supported by JST PRESTO program and KAKENHI [grant number 16K18828] for M. N. and JST CREST program for M. N., H. I. and T. I.
References
- Reed LJ. A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes. J Biol Chem. 2001;276:38329–38336.10.1074/jbc.R100026200
- Yeaman SJ. The mammalian 2-oxoacid dehydrogenases: a complex family. Trends Biochem Sci. 1986;11:293–296.10.1016/0968-0004(86)90033-2
- Kerscher L, Oesterhelt D. Pyruvate: ferredoxin oxidoreductase – new findings on an ancient enzyme. Trends Biochem Sci. 1982;7:371–374.10.1016/0968-0004(82)90118-9
- Cramer M, Myers J. Growth and photosynthetic characteristics of Euglena gracilis. Arch Mikrobiol. 1952;17:384–402.10.1007/BF00410835
- Cook JR, Heinrich B. Unbalanced respiratory growth of Euglena. J Gen Microbiol. 1968;53:237–251.10.1099/00221287-53-2-237
- Wilson B, Danforth W. The extent of acetate and ethanol oxidation by Euglena gracilis. J Gen Microbiol. 1958;18:535–542.10.1099/00221287-18-3-535
- Inui H, Miyatake K, Nakano Y, et al. Wax ester fermentation in Euglena gracilis. FEBS Lett. 1982;150:89–93.10.1016/0014-5793(82)81310-0
- Inui H, Miyatake K, Nakano Y, et al. Production and composition of wax esters by fermentation of Euglena gracilis. Agric Biol Chem. 1983;47:2669–2671.
- Inui H, Miyatake K, Nakano Y, et al. Fatty acid synthesis in mitochondria of Euglena gracilis. Eur J Biochem. 1984;142:121–126.10.1111/ejb.1984.142.issue-1
- Inui H, Ono K, Miyatake K, et al. Purification and characterization of pyruvate:NADP+ oxidoreductase in Euglena gracilis. J Biol Chem. 1987;262:9130–9135.
- Hoffmeister M, van der Klei A, Rotte C, et al. Euglena gracilis rhodoquinone: ubiquinone ratio and mitochondrial proteome differ under aerobic and anaerobic conditions. J Biol Chem. 2004;279:22422–22429.10.1074/jbc.M400913200
- Nakazawa M, Inui H, Yamaji R, et al. The origin of pyruvate:NADP+ oxidoreductase in mitochondria of Euglena gracilis. FEBS Lett. 2000;479:155–156.10.1016/S0014-5793(00)01882-2
- Inui H, Miyatake K, Nakano Y, et al. Pyruvate:NADP+ oxidoreductase from Euglena gracilis: mechanism of O2-inactivation of the enzyme and its stability in the aerobe. Arch Biochem Biophys. 1990;280:292–298.10.1016/0003-9861(90)90332-S
- Inui H, Miyatake K, Nakano Y, et al. The physiological role of oxygen-sensitive pyruvate dehydrogenase in mitochondrial fatty acid synthesis in Euglena gracilis. Arch Biochem Biophys. 1985;237:423–429.10.1016/0003-9861(85)90295-4
- Shigeoka S, Onishi T, Maeda K, et al. Occurrence of thiamin pyrophosphate-dependent 2-oxoglutarate decarboxylase in mitochondria of Euglena gracilis. FEBS Lett. 1986;195:43–47.10.1016/0014-5793(86)80126-0
- Shigeoka S, Nakano Y. Characterization and molecular properties of 2-oxoglutarate decarboxylase from Euglena gracilis. Arch Biochem Biophys. 1991;288:22–28.10.1016/0003-9861(91)90160-K
- Tokunaga M, Nakano Y, Kitaoka S. Separation and properties of the NAD-linked and NADP-linked isozymes of succinic semialdehyde dehydrogenase in Euglena gracilis z. Biochim Biophys Acta. 1976;429:55–62.10.1016/0005-2744(76)90029-2
- Oda Y, Nakano Y, Kitaoka S. Utilization and toxicity of exogenous amino acids in Euglena gracilis. J Gen Microbiol. 1982;128:853–858.
- Koren LE, Hutner SH. High-yield media for photosynthesizing Euglena gracilis z. J Protozool. 1967;14(Supplement):17.
- Nakazawa M, Andoh H, Koyama K, et al. Alteration of wax ester content and composition in Euglena gracilis with gene silencing of 3-ketoacyl-CoA thiolase isozymes. Lipids. 2015;50:483–492.10.1007/s11745-015-4010-3
- Nakazawa M, Minami T, Teramura K, et al. Molecular characterization of a bifunctional glyoxylate cycle enzyme, malate synthase/isocitrate lyase, in Euglena gracilis. Comp Biochem Physiol B Biochem Mol Biol. 2005;141:445–452.10.1016/j.cbpc.2005.05.006
- Schwab MA, Kölker S, van den Heuvel LP, et al. Optimized spectrophotometric assay for the completely activated pyruvate dehydrogenase complex in fibroblasts. Clin Chem. 2005;51:151–160.
- Oda Y, Nakano Y, Kitaoka S. Properties and regulation of valine-sensitive acetolactate synthase from mitochondria of Euglena gracilis. J Gen Microbiol. 1982;128:1211–1216.
- Huang CY, Chang AK, Nixon PF, et al. Site-directed mutagenesis of the ionizable groups in the active site of Zymomonas mobilis pyruvate decarboxylase. Eur J Biochem. 2001;268:3558–3565.10.1046/j.1432-1327.2001.02260.x
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.10.1016/0003-2697(76)90527-3
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.10.1038/227680a0
- Nakazawa M, Takenaka S, Ueda M, et al. Pyruvate:NADP+ oxidoreductase is stabilized by its cofactor, thiamin pyrophosphate, in mitochondria of Euglena gracilis. Arch Biochem Biophys. 2003;411:183–188.10.1016/S0003-9861(02)00749-X
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917.10.1139/o59-099
- Ono K, Kawanaka Y, Izumi Y, et al. Mitochondrial alcohol dehydrogenase from ethanol-grown Euglena gracilis. J Biochem. 1995;117:1178–1182.10.1093/oxfordjournals.jbchem.a124841
- Rodríguez-Zavala JS, Ortiz-Cruz MA, Moreno-Sanchez R. Characterization of an aldehyde dehydrogenase from Euglena gracilis. J Eukaryot Microbiol. 2006;53:36–42.10.1111/jeu.2006.53.issue-1
- Inui H, Ochi H, Nogami K, et al. Effect of thiamin deficiency on wax ester fermentation in Euglena gracilis. Agric Biol Chem. 1988;52:49–54.
- Tessier L, Keller M, Chan RL, et al. RNAs to pre-mature mRNAs by trans-splicing in Euglena. EMBO J. 1991;10:2621–2625.
- Kapol R, Radler F. Purification and characterization of 2-oxoglutarate decarboxylase of Leuconostoc oenos. J Gen Microbiol. 1990;136:1497–1499.10.1099/00221287-136-8-1497
- Tian J, Bryk R, Itoh M, et al. Variant tricarboxylic acid cycle in Mycobacterium tuberculosis: identification of alpha-ketoglutarate decarboxylase. Proc Natl Acad Sci USA. 2005;102:10670–10675.10.1073/pnas.0501605102
- Zhang S, Bryant DA. The tricarboxylic acid cycle in cyanobacteria. Science. 2011;334:1551–1553.10.1126/science.1210858
- Xiong W, Brune D, Vermaas WFJ. The γ-aminobutyric acid shunt contributes to closing the tricarboxylic acid cycle in Synechocystis sp. PCC 6803. Mol. Microbiol. 2014;93:786–796.10.1111/mmi.12699
- Pang SS, Duggleby RG, Schowen RL, et al. The crystal structures of Klebsiella pneumoniae acetolactate synthase with enzyme-bound cofactor and with an unusual intermediate. J Biol Chem. 2004;279:2242–2253.10.1074/jbc.M304038200
- Pang SS, Duggleby RG, Guddat LW. Crystal structure of yeast acetohydroxyacid synthase: a target for herbicidal inhibitors. J Mol Biol. 2002;317:249–262.10.1006/jmbi.2001.5419
- Wang JG, Lee PKM, Dong YH, et al. Crystal structures of two novel sulfonylurea herbicides in complex with Arabidopsis thaliana acetohydroxyacid synthase. FEBS J. 2009;276:1282–1290.10.1111/j.1742-4658.2009.06863.x
- Liu M, Sergienko EA, Guo F, et al. Catalytic acid-base groups in yeast pyruvate decarboxylase. 1. Site-directed mutagenesis and steady-state kinetic studies on the enzyme with the D28A, H114F, H115F, and E477Q substitutions. Biochemistry. 2001;40:7355–7368.10.1021/bi002855u
- Tokunaga M, Nakano Y, Kitaoka S. Subcelluar localization of the GABA-shunt enzymes in Euglena gracilis strain Z. J Protozool. 1979;28:471–473.10.1111/jeu.1979.26.issue-3
- Tomita Y, Yoshioka K, Iijima H, et al. Succinate and lactate production from Euglena gracilis during dark, anaerobic conditions. Front Microbiol. 2016;7:1–8.
