Abstract
One new (1, SZMT01) and one known (2) anti-aging substances were isolated from Shenzhou honey peach fruit. Their structures were elucidated by spectroscopic methods and chemical derivatization, and the result reveals that these two compounds are sesquiterpene glucosides. SZMT01 possesses a new glycosylation with an ester linkage at one terminal in an acyclic sesquiterpenoid which is the end of a double bond at another terminal. Both compounds extend the replicative lifespan of K6001 yeast strain at doses of 7.5 and 25 μM. Then, to understand the action mechanism involved, we performed an anti-oxidative experiment on SZMT01. The result revealed that treatment with SZMT01 increased the survival rate of yeast under oxidative stress. Moreover, the lifespans of sod1 and sod2 mutant yeast strains with a K6001 background were not affected by SZMT01. These results demonstrate that anti-oxidative stress performs important roles in anti-aging effects of SZMT01.
Human longevity had markedly increased due to the development of medical levels and technology during the past decades. Therefore, a rapid aging population is relatively common in modern society, and the increase in age-related diseases prevalence such as Alzheimer’s and Parkinson’s diseases and diabetes, is becoming a considerable social economic challengeCitation1) and a severe threat to human health in an aging society. Although numerous commercially available drugs are used for treating these diseases,Citation2) these drugs can only alleviate clinical symptoms and cannot cure the diseases essentially. Thus, anti-aging will be a promising strategy to prevent the occurrence of aging-related diseases.
Peach is a kind of deciduous tree native to South Asia and cultivated worldwide. Shenzhou honey peach (Prunus persica L. Batsch cv. Shenzhou)Citation3) is a unique peach characterized by its shape, taste, and fragrance because of the special geographical environment and soil conditions. For more than 2000 years, Shenzhou peach called “GongTao” was sent to the palace as royal tribute and always offered as birthday congratulatory gift. Peach is an ancient fruit that is known to prolong life according to the sentence “Eating peach can make you immortal” in “Shen Nong’s Herbal Classic.” Thus, we investigated Shenzhou peach from Shenzhou City, Hebei Province to search for the material basis for its anti-aging activity.
Yeast is a well-known bioassay model in anti-aging research because of its characteristics of short generation time, genetic tractability, and low cost.Citation4) Replicative aging is used to evaluate the longevity of yeast. The traditional replicative lifespan assay often uses a micromanipulator to remove the daughter cells produced by one mother cell for every 2 h and became a rate-limited step in the progress of aging research. In 2004, Jarolim et al. established the replicative lifespan assay with the K6001 yeast strain to improve the lifespan assay.Citation5) Using this bioassay system, we have isolated many anti-aging substances from natural products.Citation6–9)
With the guidance of the same bioassay system, one new compound SZMT01 (1, SZMT is short for Shenzhou Mitao (Shenzhou honey peach in Chinese)) and one known compound (2) were isolated from Shenzhou honey peach (Fig. ). The two compounds can significantly extend the replicative life span of yeast. In addition, studies on the action mechanism of SZMT01 suggest that anti-oxidative stress performs important roles in the anti-aging effects of SZMT01.
Materials and methods
General experimental procedures
Preparative high-performance liquid chromatography (HPLC) was performed on an HPLC system equipped with ELITE P-230 pumps. High-resolution electrospray ionization mass spectroscopy (HRESIMS) measurements were recorded on Agilent Technologies 6224A accurate mass time of flight (TOF) LC/MS system. NMR spectra were recorded on a Bruker AV III-500 spectrometer, and NMR chemical shifts in δ (ppm) were referenced to solvent peaks of δC 49.0 and δH 3.30 for CD3OD. The optical rotations were measured on a JASCO P-1030 digital polarimeter.
Plant material
Shenzhou honey peach fruits were harvested and collected from Shenzhou City, Hebei Province, China in September 2015, and a voucher specimen (No. 20150912) was preserved in Zhejiang University, Institute of Materia Medica.
Extraction and isolation
The peach fruits were extracted (3.0 kg, fresh weight) with methanol for 24 h with shaking. The extract was filtered and concentrated to obtain a brown viscous oil (100 g), which was chromatographed on an ODS open column (Cosmosil 75 C18-OPN, Nacalai Tesque) and eluted with a MeOH/H2O step gradient (30:70, 50:50, 70:30, 100:0, by volume), to afford four fractions. The active sample (700.0 mg) eluted with MeOH/H2O (70:30) was then separated through ODS open column again with a more detailed MeOH/H2O step gradient (50:50, 55:45, 60:40, 65:35, 70:30, 80:20, 100:0, by vol.), to obtain eight fractions. The active sample (3.7 mg) eluted with MeOH/H2O (60:40) was subjected to HPLC [Develosil PAKC18, (10 × 250 mm), flow rate: 3 mL/min, 63% aq. MeOH] to yield 1 (1.1 mg, tR = 13.9 min) as the active substance. Another active sample (2.3 mg) eluted by the second ODS open column with MeOH/H2O (65:35) was subjected to HPLC [Develosil PAKC18, (10 × 250 mm), flow rate: 3 mL/min, 65% aq. MeOH] to yield compound 2 (1.8 mg, tR = 14.5 min) as the active substance.
Hydrolysis of 1 and the sugar analysis
Compound 1 (50 μg) was dissolved in 400 μL MeOH and then hydrolyzed by saturated Na2CO3 overnight. The reaction solution was concentrated, dried up, and then partitioned between EtOAc and H2O, and the H2O layer was dried in vacuo and redissolved in pyridine (25 μL) containing 5 mg/mL L-cysteine methyl ester. The reaction was heated at 60 °C for 1 h. A 25 μL solution of o-tolylisothiocyanate (5 mg/mL) in pyridine was added to the mixture, which was heated at 60 °C for 1 h to obtain an aldose o-tolylthiocarbamate derivative. Meanwhile, the aldose thiocarbamate standards were prepared as follows: L, D-glucoses were derivatized with L-cysteine methyl ester and o-tolylisothiocyanate according to the reported procedure.Citation10) The reaction mixture was dried up, redissolved in methanol, and directly analyzed by Agilent Technologies 6224A accurate mass TOF LC/MS under the following conditions: Agilent Extend C18 column (3.5 μm, 3.0 mm × 100 mm); detected at 210 nm; t = 0 min MeOH/H2O/formic acid (30:70:0.1), t = 25 min MeOH/H2O/formic acid (95:5:0.1); and flow rate: 0.45 mL/min.
D–glucose was identified as the sugar moiety in SZMT01 by the comparison of the retention time of the aldose thiocarbamate derivative (tR = 12.22 min) with those of the aldose thiocarbamate standards, namely, L-cysteine-D-glucose (tR = 12.26 min) and L-cysteine-L-glucose (tR = 12.78 min).
SZMT01 (1)
Amorphous solid; [α]28 D −29.1 (c 0.16, MeOH); For 1H and 13C NMR, see Table ; HRESIMS m/z 437.2151 [M + Na]+, calcd. for C21H34O8Na+ 437.2151.
Table 1. 1H NMRTable Footnotea and 13C NMRTable Footnoteb data for SZMT01 (1) in CD3OD (δ in ppm, J in Hz).
Compound 2
Amorphous powder; [α]28 D −18.0 (c 0.2, MeOH); HRESIMS m/z 423.2368 [M + Na]+, calcd. for C21H36O7Na+ 423.2359.
Yeast strains, culture media, and lifespan assay
Wild-type BY4741, K6001 with a W303 background, and sod1 and sod2 mutations with a K6001 background were employed. The lifespan assay was performed as described in the previous study.Citation11) The K6001 yeast strain was cultured in YPGal medium consisting of 3% (w/v) galactose, 1% (w/v) yeast extract powder, and 2% (w/v) polypeptone, or on YPD that contains 2% (w/v) glucose in place of galactose. To prepare agar plates, 2% (w/v) agar was added to the medium. For biological activity test, the K6001 yeast strain was inoculated into a 5 mL of galactose medium and incubated in a shaking incubator with the temperature of 28 °C and rotational speed of 160 rpm. When cells were grown to logarithmic phase, 1 mL of yeast culture media was centrifuged at 1500 rpm for 3 min to obtain the yeast pellet, which was washed for three times and diluted with phosphate buffer solution (PBS). At the same time, samples were dissolved in 97% ethanol and smeared the corresponding amount of compound on agar plate according to concentration. To make ethanol volatilize, these plates were put in the clean bench. Then 4000 cells were spread on glucose agar plates containing different concentrations of the samples. The plates were incubated in an incubator at 28 °C for two days. The daughter cells of 40 microcolonies formed on the agar plate were randomly observed under a microscope and the number of daughter cells around each mother cell was counted. We calculated the average value of the daughter cells produced by 40 microcolonies as average lifespan. The survival curve of replicative lifespan was made according to the percentage of each generation. The bioassay method of replicative lifespan of sod1 and sod2 mutants were identical to that of the K6001 strain.
Anti-oxidative stress assay
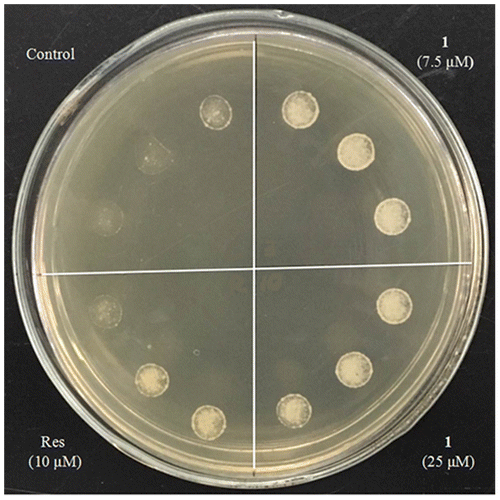
BY4741 yeast was treated with resveratrol (10 μM) as a positive control or SZMT01 (7.5 and 25 μM) at 28 °C and 160 rpm in a shaker overnight. Subsequently, yeast cultures with the same OD value in each group were spotted on agar plates containing 9 mM H2O2. The growth of yeast on the plate was observed and photographed after incubation at 28 °C for three days.
Statistical analysis
The significant differences among groups in all experiments were determined by ANOVA, followed by two-tailed multiple t-tests with Bonferroni correction using SPSS biostatistics software. The p value of less than 0.05 was considered statistically significant.
Results and discussion
Isolation
A MeOH extract of Shenzhou honey peach (Prunus persica L. Batsch cv. Shenzhou) was chromatographed on an ODS open column twice to obtain two active fractions. These fractions were purified by HPLC to yield SZMT01 (1, 0.00004% of fresh weight) and compound 2 (0.00006% of fresh weight), respectively.
Structure elucidation
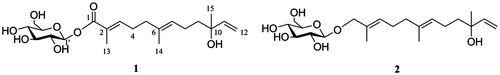
SZMT01 (1) possesses the molecular formula C21H34O8, as determined by HRESIMS measurement. The 1H NMR data of 1 show the presence of three methyl groups at δH 1.24 (3H, s), 1.62 (3H, s), and 1.84 (3H, s), as well as five olefinic protons at δH 6.68 (1H, t, J = 7.4 Hz), 5.90 (1H, dd, J = 17.4, 10.8 Hz), 5.18 (1H, dd, J = 17.4, 1.4 Hz), 5.16 (1H, t, J = 7.7 Hz), and 5.02 (1H, dd, J = 10.8, 1.4 Hz), and four methylenes at δH 2.32 (2H, m), 2.11 (2H, t, J = 7.4 Hz), 2.02 (2H, m), and 1.51 (2H, m) (Table ). The 1H NMR spectrum also displayed the signals of a hexose at δH 5.52 (1H, d, J = 7.8 Hz), 3.82 (1H, dd, J = 11.7, 1.6 Hz), 3.67 (1H, dd, J = 11.7, 4.7 Hz), 3.43 (1H, m), 3.38 (1H, m), and 3.36 (2H, m). The 13C NMR spectra of 1 revealed the presence of 21 carbon signals caused by one carbonyl carbon (δC 168.2), three double bonds (δC 146.3, 145.3, 134.9, 128.3, 126.7, and 112.0), three methyl groups (δC 27.6, 15.9, and 12.4), an oxyquaternary sp3 carbon (δC 73.8), four methylene groups (δC 43.3, 39.2, 28.4, and 23.7) as well as the sugar moiety (δC 96.0, 78.8, 78.2, 74.1, 71.2, and 62.4). The 1H NMR and 13C NMR data suggest that the compound possesses a sesquiterpenoid skeleton.
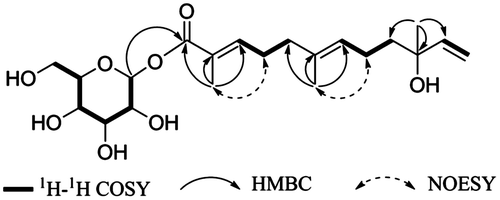
A 1H–1H COSY spectrum analysis of 1 resulted in a depiction of partial structures (in bold bonds in Fig. ). In the HMBC spectrum of SZMT01, the partial structures are connected to yield a gross structure, as follows: methyl protons (H-13) to C-1, C-2, and C-3; methyl protons (H-14) to C-5, C-6, and C-7; and methyl protons (H-15) to C-9, C-10, and C-11. Similarly, the HMBC correlations of anomeric proton H-1′ with C-1 indicate the location of the sugar moiety in 1.
To determine the sugar type and absolute stereochemistry of the sugar moiety, we prepared an aldose o-tolylthiocarbamate derivative and compared the compound with derivatized authentic standards by LC/HRESIMS, which reveals the presence of D-glucose in 1. The signal of an anomeric H-1′ appeared at δH (5.52, 1H, d, J = 7.8 Hz), suggesting the β configuration of the sugar moiety. These data suggested that 1 is a kind of sesquiterpene glucoside.
The stereostructure of the double bond was determined by the NOESY correlations. The correlations between H-13 and H-4; and between H-14 and H-8, which establish the trans configuration of the double bonds. Position 10 is a chiral center connected with a long flexible chain, a tertiary hydroxyl, a terminal vinyl, and a methyl groups. Methods which can be applied to determine such kind of stereochemistry of natural products need to be developed. Therefore, the stereochemistry of position 10 remains to be clarified. Hence, SZMT01 was determined to be (2E,6E)-10-hydroxy-2,6,10-trimethyldodeca-2,6,11-trienoic acid β-D-glucose ester, a new glycosylation with an ester linkage at one terminal in an acyclic sesquiterpenoid which is the end of a double bond at another terminal.
Compound 2 possesses the molecular formula C21H36O7, as determined by HRESIMS measurement. The 13C NMR (125 MHz, in CD3CD): δC 14.1 (C-13), 16.0 (C-14), 23.7 (C-8), 27.4 (C-4), 27.6 (C-15), 40.3 (C-5), 43.4 (C-9), 62.8 (C-6′), 71.8 (C-4′), 73.9 (C-10), 75.1 (C-2′), 76.0 (C-1), 77.9 (C-5′), 78.2 (C-3′), 102.6 (C-1′), 112.0 (C-12), 126.0 (C-7), 129.9 (C-3), 132.9 (C-2), 135.7 (C-6), and 146.4 (C-11). The 1H NMR and 13C NMR data were identified as previously reported one.Citation12) Thus, compound 2 was determined to be (2E,6E)-2,6,10-trimethyl-2,6,11-dodecatriene-1,10-diol-1-O-β-D-glucopyranoside.
Anti-aging activities of 1 and 2
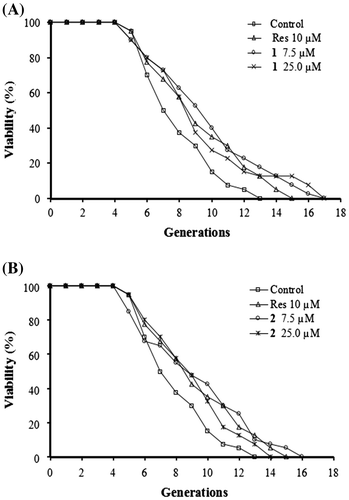
In the bioassay system, resveratrol was used as a positive control to evaluate the reliability of the system. Resveratrol is well known to exhibit anti-aging effects in numerous organisms, including yeast strains.Citation13) In the K6001 bioassay system, the most effective dose was 10 μM, as reported in a previous study.Citation11) Then, the biological activity of compounds 1 and 2 at doses of 7.5 and 25 μM was evaluated using the K6001 yeast strain. As shown in Figs. at both doses extended the replicative lifespan of K6001 (p < 0.05, p < 0.01) in comparison with resveratrol at 10 μM (p < 0.05). These results demonstrate that SZMT01 (1) and 2 exhibit anti-aging activity.
Fig. 3. Effects of 1 (A) and 2 (B) on the replicative lifespan of K6001 yeast strain. The average lifespan of K6001 was as follows: Control, 7.10 ± 0.34; Res (resveratrol, positive control) at 10 μM, 8.40 ± 0.47*; 1 at 7.5 μM, 8.87 ± 0.53**, 1 at 25 μM, 8.37 ± 0.51*; 2 at 7.5 μM, 8.40 ± 0.54*, 2 at 25 μM, 8.20 ± 0.41*. Each experiment was repeated thrice and the results were displayed as mean ± SEM.
SZMT01 (1) improves the oxidative resistance of yeast
Oxidative stress is a negative effect produced by free radicals, and it is considered to be one of the key factors for aging. Oxidative free radicals deleteriously damages biological molecules like DNA, proteins, and lipids.Citation14) Therefore, we focused on this point to measure whether SZMT01 could improve the oxidative resistance of yeast. The result in Fig. demonstrates that treatment with SZMT01 at 7.5 and 25 μM significantly improves the survival rate of yeast under oxidative stress conditions caused by 9 mM H2O2. This result indicates that SZMT01 presents anti-oxidative activity. Antioxidants such as resveratrol showed similar activity even in yeast, nematode, and mouse systems.Citation15)
Fig. 4. Effects of 1 on the oxidative resistance of yeast. BY4741 yeast was incubated for 2 days after treatment with 1 at 7.5, 25 μM or Res (resveratrol, positive control) at 10 μM; then about 5 μL yeast culture with the same OD was dropped onto glucose medium agar plates containing 9 mM H2O2. The yeast was incubated for 3 days at 28 °C and photographed.
SZMT01 (1) does not affect the lifespans of sod1 and sod2 mutants with a K6001 background
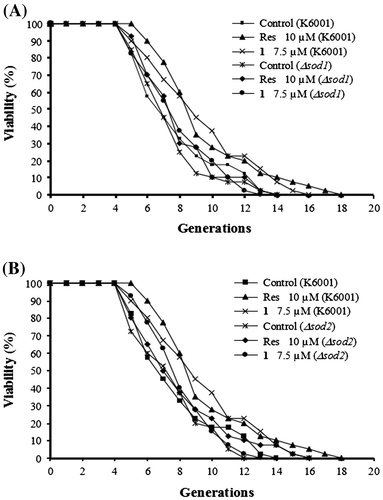
Oxidative stress plays an important role in aging, and SOD (superoxide dismutase) are important proteins that participate in regulation of oxidative stress. To determine whether SOD gene took part in the extending lifespan activity of SZMT01, we employed sod1 and sod2 mutants with a K6001 background to examine the activities of SZMT01 on replicative lifespan. The lifespan of sod1 and sod2 mutants was shorter than that of K6001 yeast strains, and SZMT01 did not affect the replicative lifespan of these mutants (Fig. ). These results indicated that the SOD gene was involved in the anti-aging effect of SZMT01.
Fig. 5. Effects of 1 on the replicative lifespan of sod1 (A), sod2 (B) mutant yeast strains with a K6001 background. The daughter cells of 40 microcolonies of each plate were counted randomly. The assay was repeated at least thrice. The results were displayed as mean ± SEM. The average lifespan of untreated K6001 was 6.85 ± 0.42 generations; Res (resveratrol, positive control) at 10 μM, 8.70 ± 0.48**; and 1 at 7.5 μM, 8.40 ± 0.48*.
In the present study, one new (1, SZMT01) and one known (2) anti-aging sesquiterpene glucosides were isolated from Shenzhou honey peach. SZMT01 possesses a new glycosylation with an ester linkage at one terminal in an acyclic sesquiterpenoid which is the end of a double bond at another terminal, while 2 has a glycosylation with an ether linkage at one terminal of an acyclic sesquiterpenoid. The biological activity results showed that the position and type of linkage group of the glycosylation did not significantly affect anti-aging activity of these two compounds. In addition, anti-oxidative assay and sod1 and sod2 mutants lifespan assay were carried out to investigate brief mechanism of action of 1. The data from these experiments suggested that SZMT01 increased the survival rate of yeast under oxidative stress, and SOD1, SOD2 are essential genes for lifespan extension of SZMT01.
In conclusion, SZMT01 and 2, two sesquiterpene glucosides from Shenzhou honey peach showed anti-aging effects on K6001 yeast strain and the mechanism study showed anti-oxidative stress and SOD1, SOD2 play important roles in anti-aging effects of SZMT01. Shenzhou honey peach is worthy of further investigation, including evaluation of anti-aging activity using higher organisms and intensive studies on mechanism of action of active components.
Funding
This work was supported by government of Shenzhou city of Hebei Province and partial supported by International Science and Technology Cooperation Program of China [grant number 2014DFG32690]; NSFC [grant number 21661140001], [grant number 81273385]; Doctoral Fund of Ministry of Education [grant number 20130101110093].
Author contribution
Jianhua Qi, Lan Xiang, and Hiroyuki Osada designed and guided experiments, and revised the manuscript. Yanhui Wang and Yanfei Lin performed the experiments, and analyzed the data. Yanhui Wang wrote the draft of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgment
We thank Mr. Zhanguang Zhang for providing us Shenzhou honey peach as the experimental material. We are grateful to M. Breitenbach, Salzburg University, Austria for gifts of K6001 yeast strain, and wild-type BY4741, sod1 and sod2 mutation with a K6001 background were kindly provided by Professor Akira Matsuura.
Notes
Abbreviations: HPLC, high-performance liquid chromatography; HRESIMS, high resolution electrospray ionization mass spectroscopy; TOF, time of flight; PBS, phosphate buffer solution; SOD, superoxide dismutase; COSY, correlated spectroscopy; HMBC, heteronuclear multiple bond correlation; NMR, nuclear magnetic resonance; NOE, nuclear overhauser enhancement.
References
- Vaiserman AM, Lushchak OV, Koliada AK. Anti-aging pharmacology: promises and pitfalls. Ageing Res Rev. 2016;31:9–35.10.1016/j.arr.2016.08.004
- Lleó A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer's disease. Annu Rev Med. 2006;57:513–533.10.1146/annurev.med.57.121304.131442
- Wu Q, Wang D, Li B, et al. Effects of ethylene regulators on storage quality and physiological characteristics of ‘Shenzhou honey’ peaches. J Agr Univ Hebei. 2016;39:75–80.
- Parrella E, Longo VD. The chronological life span of Saccharomyces cerevisiae to study mitochondrial dysfunction and disease. Methods. 2008;46:256–262.10.1016/j.ymeth.2008.10.004
- Jarolim S, Millen J, Heeren G, et al. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004;5:169–177.10.1016/j.femsyr.2004.06.015
- Xiang L, Sun KY, Lu J, et al. Anti-aging effects of phloridzin, an apple polyphenol, on yeast via the SOD and Sir2 genes. Biosci Biotechnol Biochem. 2011;75:854–858.10.1271/bbb.100774
- Sun KY, Cao SN, Pei L, et al. A steroidal saponin from Ophiopogon japonicus extends the lifespan of yeast via the pathway involved in SOD and UTH1. Int J Mol Sci. 2013;14:4461–4475.10.3390/ijms14034461
- Sun YJ, Lin YF, Cao XL, et al. Sterols from Mytilidae show anti-aging and neuroprotective effects via anti-oxidative activity. Int J Mol Sci. 2014;15:21660–21673.10.3390/ijms151221660
- Lin YF, Sun YJ, Weng YF, et al. Parishin from Gastrodia elata extends the lifespan of yeast via regulation of Sir2/Uth1/TOR signaling pathway. Oxid Med Cell Longev. 2016;2016:1–11. DOI:10.1155/2016/4074690
- Tanaka T, Nakashima T, Ueda T, et al. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem Pharm Bull. 2007;55:899–901.10.1248/cpb.55.899
- Weng YF, Xiang L, Matsuura A, et al. Ganodermasides A and B, two novel anti-aging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorg Med Chem. 2010;18:999–1002.10.1016/j.bmc.2009.12.070
- Xi FM, Li CT, Han J, et al. Thiophenes, polyacetylenes and terpenes from the aerial parts of Eclipata prostrata. Bioorg Med Chem. 2014;22:6515–6522.10.1016/j.bmc.2014.06.051
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506.10.1038/nrd2060
- Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006.10.1039/C4RA13315C
- Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689.10.1038/nature02789