?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Soybean cyst nematode (SCN) Heterodera glycines Ichinohe, a plant parasite, is one of the most serious pests of soybean. In this paper, we report that SCN is attracted to nitrate and its analogs. We performed attraction assays to screen for novel attractants for SCN and found that nitrates were attractants for SCN and SCN recognized nitrate gradients. However, attraction of SCN to nitrates was not observed on agar containing nitrate. To further elucidate the attraction mechanism in SCN, we performed attraction assays using nitrate analogs (,
,
). SCN was attracted to all nitrate analogs; however, attraction of SCN to nitrate analogs was not observed on agar containing nitrate. In contrast, SCN was attracted to azuki root, irrespective of presence or absence of nitrate in agar media. Our results suggest that the attraction mechanisms differ between plant-derived attractant and nitrate.
Nitrate and its analogs regulate the attraction of soybean cyst nematode.
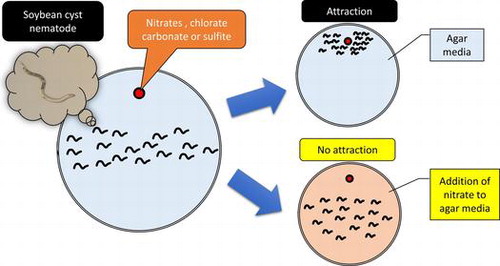
Soybean cyst nematode (SCN) Heterodera glycines Ichinohe is a root parasite, which can cause serious damage to various legume crops. It is reported that the annual crop losses in soybean fields in the USA are estimated at 1.3 billion US dollars.Citation1) In addition, SCN causes approximately 10–20% yield loss in China.Citation2) The SCN life cycle includes an egg stage, four juvenile stages, and an adult stage. The first step in its life cycle encompasses hatching from eggs in response to the hatching stimulants released from the host plant. Subsequent to hatching, the second-stage juveniles (J2s) migrate to the host root, in response to the host-derived attractants. After infection, J2s become sedentary, molt three times in the host plant, and develop into either male or female adults. Adult males move out of the roots to search for adult females and to fertilize eggs. Adult females holding fertilized eggs in their body die and their outer body layers harden to form a protective cyst. Encysted eggs are resistant to environmental stresses and remain dormant in the soil. Therefore, it is difficult to protect crops from SCN damage although many approaches for SCN control have been explored. Currently, various methods, such as the use of resistant varieties, nematicides, and inclusion of antagonistic crops in crop rotation, have been used in an attempt to control SCN damage.Citation3) Development of SCN-resistant varieties, in particular, has proven to be a highly effective strategy, and their resistance genes have been isolated.Citation4,5) However, it has been observed that SCNs capable of infecting the SCN-resistant varieties have emerged.Citation6) Thus, novel methods of SCN control are necessary.
Almost all organisms, including insects and nematodes, use chemicals as communication signals with their environment and other organisms. For example, ascarosides, a group of small molecular pheromones conserved in nematodes, regulate species-specific behaviors, including retention, avoidance, and long-range attraction.Citation7) The idea of using such behavior-modifying chemicals for the management of harmful insects in agriculture was conceived over 50 years ago.Citation8)
Some gases, chemicals and ions act as attractants for plant-parasitic nematodes. Many plant-parasitic nematodes, including Meloidogyne incognita and Heterodera schactii, are attracted by a steep CO2 gradient.Citation9) Lauric acid isolated from crown daisy root exudate mediates the chemotaxis of M. incognita through Mi-flp-18 which encodes a peptide neuromodulator.Citation10,11) Tylenchulus semipenetrans prefers sodium acetate (CH3COONa) and potassium acetate (CH3COOK), whereas M. javanica prefers sodium carbonate (Na2CO3) and sodium bicarbonate (NaHCO3), respectively, to CH3COOK, as attractants.Citation12) On the other hand, Globodera. rostochiensis and M. incognita are attracted by ammonium nitrate (NH4NO3) and potassium nitrate (KNO3), respectively.Citation13,14) In the case of attractants for SCN, while Papademetrious and Bone reported that ZnSO4 and MgCl2 attracted SCN,Citation13) Beeman et al. reported that NH4NO3 and KNO3 also attract SCN.Citation15) However, the mechanism underlying this attraction is not known. In addition, although it is suggested that host-derived chemicals modulate SCN behavior, these chemicals have not been identified.
In this study, to unveil the mechanism of SCN attraction, we screened for chemicals that regulate SCN behavior by performing attraction assay and found that not only NH4NO3 and KNO3 but also nitrate analogs are attractants for SCN, in vitro.
Materials and methods
Chemicals. Inorganic salts used in this study were purchased from the Tokyo Chemical Industry (Tokyo, Japan), Wako pure chemical (Osaka, Japan), and Sigma–Aldrich (St Louis, MO, USA). Glycinoeclepin A was provided by from Prof. Keiji Tanino (Hokkaido University, Japan).Citation16)
Nematodes. SCN infested soil was the gift from Dr. Atsuhiko Kushida (NARO Hokkaido Agricultural Research Center, Japan). The H. glycines population was maintained in the greenhouse on SCN-susceptible soybean “Kitamusume.” J2s were collected according to a previously described method.Citation17) SCN cysts were collected from the soil in which SCN-infected soybean was grown. Soil was washed with water and the suspension was passed through a 450 μm-pore sieve three times, to remove the soil. Cysts and the soil remaining on the sieve were washed with water and transferred to filter paper. Collected cysts were opened manually with a needle, in a drop of water, under a microscope. Eggs were rinsed 10 times with deionized water and the egg suspension was placed in the wells of a 96-well plate (approximately 300 eggs/well). After a glycinoeclepin A solution (final concentration: 1 nM) was added to each well, the plate was incubated in a chamber at 25 °C for 5 days. Hatched J2s were washed three times with water.
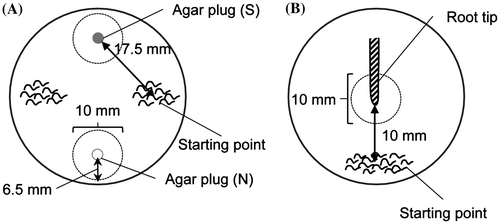
Attraction assays. Attraction assays using agar plug were performed according to a previously described method, with a slight modification.Citation18) This assay was performed in 6-well tissue culture plates containing 5 mL of agar medium (0.7% agar and 10 mM Tris-HCl (pH 7.2)) per well. We used agar media supplemented with plant growth salts (Murashige and Skoog salt, Gamborg’s B5 salt, or minimal medium salt) or KNO3 if required. Citation19−21) The model of attraction is shown in Fig. (A). Agar plugs containing 2% agar and inorganic salts (S) were placed on agar surface, at 6.5 mm from the edge of the well. As a control, agar plug containing only 2% agar (N) was placed directly opposite S. Approximately 50 hatched J2s were placed on two positions on agar surface (Starting point). Then, assay plates were incubated at 25 °C in dark conditions. After 1, 3, 5, and 7 h, the number of nematodes that moved to the area within 5 mm of the agar plug on all directions was counted. The chemotaxis index (CI) was calculated as:
Fig. 1. The models of attraction assay using agar plug.
(the number of nematodes within the area around S – the number of nematodes within the area around N)/total number of nematodes on the assay plate.
We used eight salts (500 mM NaNO3, Na2SO4, NaCl, NaH2PO4, KCl, NH4Cl, CuSO4 and ZnSO4), five nitrates (500 mM KNO3, NaNO3, Ca(NO3)2, Mg(NO3)2 and NH4NO3) or four nitrate analogs (500 mM NaClO3, KClO3, K2SO3 and K2CO3), in attraction assays using agar plug.
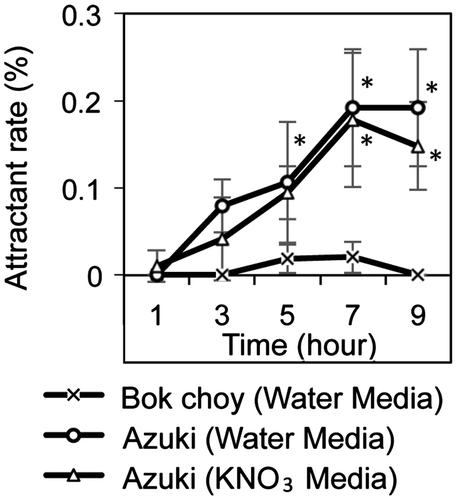
Attraction assays using azuki and bok choy root tip were performed in 6-well tissue culture plates containing 5 mL of agar medium (0.7% phyto agar with or without 3 mM KNO3) per well. Azuki bean (Vigna angularis cultivar “erimo-azuki”) seeds were purchased from Daimasu. (Tokyo, Japan). Bok choy (Brassica rapa cultivar “Qinggengcai”) seeds were purchased from SAKATA SEED CORPORATION (Kanagawa, Japan). Azuki bean and bok choy seeds were sterilized in 2.5% NaOCl solution containing 0.05% Tween-20 for 10 min, and washed 10 times with water. Sterilized seeds were placed on a 90 mm petri plate containing 0.7% agar and incubated at 25 °C under light condition. After 7 days, azuki and bok choy seedlings were cut at approximately 10 mm from root tips. A single root tip was placed on an assay plate as described in Fig. (B). About 50 hatched J2s were placed on the plate (Starting point, as shown in Fig. (B)). Then, the assay plate was incubated at 25 °C in dark conditions. After 1, 3, 5, 7, and 9 h, the number of nematodes that moved to the area within 5 mm of the root tip on all directions (dotted circle) was counted. Attractant rate was calculated as:
Number of nematodes within the dotted circle/total nematodes on plate.
Measurement of nitrate concentration in agar medium. We measured nitrate concentration in the same agar medium as described above. Measurement of nitrate concentration was performed according to a previously described method, with a slight modification.Citation22,23) Agar blocks were cut out at the distance of 3.5, 8.5, and 17 mm from agar plug, respectively. 500 μL of water was added, mixed thoroughly and filtrated by 0.45 μm-pore membrane. About 2 μL of 1 N HCl was added to 100 μL of filtrate. Absorbance at 220 nm (A220) and 275 nm (A275) were measured by V730Bio spectrophotometer (JASCO, Tokyo, Japan). Nitrate concentration was calculated from standard carve prepared in the range of 10–100 μM KNO3. For samples and standards, we subtracted two times A275 from A220 to obtain the absorbance due to nitrate.
Results
Screening for attractants for SCN
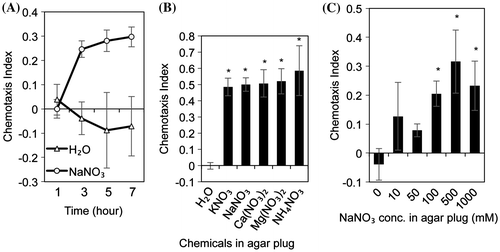
Some salts regulate SCN behavior. In the present study, we screened for novel attractants using eight salts (NaNO3, Na2SO4, NaCl, NaH2PO4, KCl, NH4Cl, CuSO4 and ZnSO4). According to the results of the screening, SCN showed strong attraction to 500 mM NaNO3 (Supplemental Fig. S1). On the other hand, ZnSO4, which is previously reported as attractant for SCN, did not induce SCN attraction.Citation13) In our experimental condition, the attraction to NaNO3 at 3-, 5-, and 7-h post-exposure was significantly higher than at 1-h post-exposure (Fig. (A)). Therefore, we selected the 3-h post-exposure for the attraction assay in the subsequent experiments. Because SCN was attracted by NH4NO3 and KNO3Citation15) we hypothesized that nitrates elicited SCN attraction. To examine the nitrate-dependent attraction, we performed attraction assays using various nitrates (KNO3, NaNO3, Ca(NO3)2, Mg(NO3)2, and NH4NO3) (Fig. (B)). SCN showed a strong attraction to all nitrates with significant differences. These results indicate that nitrates act as attractants for SCN.
Fig. 2. Attraction assays using nitrates.
SCN attraction is dependent on nitrate concentration gradients
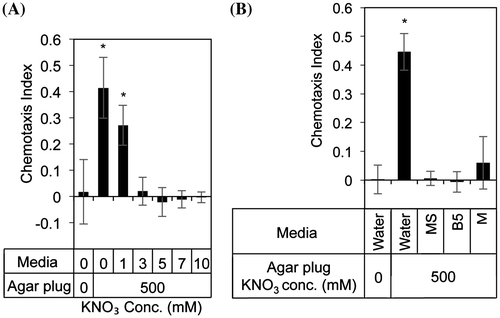
Because the nematodes responded to a gradient of attractants, we estimated the nitrate concentration dependency. SCN attraction to agar plug was significant with NaNO3 concentrations over 100 mM (Fig. (C)). In addition, we calculated the correlation between the nitrate concentration and distance from the agar plug using linear regression (Pearson correlation coefficient = 0.94–0.99, Table ). Nitrate concentrations in agar media changed depending on both the distance from the agar plug and nitrate concentration on the agar plug (Table , Supplemental Figs. S2 and S3). In addition, nitrate concentration in agar media was relatively lower than that in agar plug. Nitrate gradients were 2.92, 11.7, 28.1, 113, and 259 μM/mm for agar plugs with nitrate concentrations of 10, 50, 100, 500, and 1000 mM, respectively. These results indicate that SCN can perceive a moderate nitrate gradient. We used -containing agar plug as a positive control for subsequent study, because KNO3 is easy to make agar plug in comparison with NaNO3.
Table 1. Nitrate gradient in assay plate.
Nitrate-dependent attraction is inhibited on agar medium containing nitrates
In the natural environment, nitrates are distributed in the soil. To estimate the effects of nitrates in the soil on SCN behavior, we added nitrates to agar media to mimic the soil condition and performed the attraction assays. Attraction of SCN to nitrate-containing agar plug attenuated on adding KNO3 to the agar medium, in a concentration-dependent manner (Fig. (A)). The addition of salt mixtures used as plant growth media (Murashige & Skoog salt mixture, Gamborg’s B5 salt mixture, and minimal medium salt mixture) also inhibited the attraction of SCN to nitrate-containing agar plug (Fig. (B)). The inhibitory activities of these media were dependent on the nitrate concentration (Murashige & Skoog medium, Gamborg’s B5 medium, and minimal medium contain approximately 39, 25, and 3.2 mM nitrate, respectively). However, adding nitrates to agar media did not affect the nitrate-gradient in agar media (Supplemental Fig. S4). These results suggest that adding nitrates over 3 mM on agar media cancels the effect of nitrate gradient on SCN attraction.
Fig. 3. Attraction assay using nitrate on -containing media.
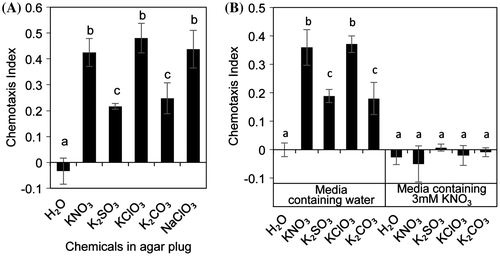
Nitrate analogs are attractants against SCN
To elucidate the attraction mechanism in SCN, we estimated the effects of nitrate analogs on SCN attraction. We chose chlorate (), sulfite (
), and carbonate (
) as nitrate analogs. Remarkably, SCN was attracted to agar plugs containing nitrate analogs (Fig. (A)). Nitrate and chlorate, in particular, showed higher CI than did sulfite and carbonate. However, this attraction was attenuated by the addition of nitrate (3 mM) in agar media (Fig. (B)).
Fig. 4. Attraction assay using nitrate analogs.
Plant-derived attractants are different from nitrate and its analogs
SCN is attracted to plant by unknown chemical signal(s). Next, we checked whether nitrate is a plant-derived attractant (Fig. ). The root tip of azuki bean, which is the host of SCN, attracted SCN. On the other hand, the root tip of bok choy, which is the non-host of SCN, did not attracted SCN. Furthermore, the addition of KNO3 (3 mM) in agar media did not affect SCN attraction to azuki root.
Discussion
In this study, we screened for the chemicals that regulate SCN behavior, and found that nitrate analogs (chlorate, sulfite, and carbonate) were attractants for SCN. On the other hand, ZnSO4 did not induce SCN attraction, though Papademetrious and Bone had previously reported that these salts were attractant for SCN (Supplemental Fig. S1).Citation13) Differences between our results and reported literature might be attributed to the differences in the assay methods. As shown in Fig. (C), the CI of the agar plug containing 1000 mM NaNO3 was slightly lower than that of the agar plug containing 500 mM NaNO3. This phenomenon was also observed when we performed attraction assays using agar plugs containing various concentrations of KNO3 (Supplemental Fig. S5). Recently, Hida et al. reported that chemotaxis of M. incognita to KNO3 is dependent on the concentration gradient.Citation14) That is, KNO3 functioned as an attractant and a repellent at relatively low and high concentration gradients, respectively. These results might suggest the existence of a similar mode of action regarding nitrates in SCN and M. incognita. As shown in Figs. and , we added nitrate to agar media and performed the attraction assays. Surprisingly, attraction of SCN to nitrate was significantly decreased in agar media containing more than 3 mM nitrate. These results suggest that adding nitrates over 3 mM on agar media cancels the effect of nitrate gradient on SCN attraction.
As shown in Fig. , nitrates elicited SCN attraction. To elucidate the attraction mechanism in SCN, we estimated the effects of nitrate analogs on SCN attraction (Fig. ). We chose chlorate, sulfite, and carbonate as nitrate analogs, because the structure of these ions is similar to that of nitrate; chlorate and sulfite have a trigonal pyramid structure, whereas nitrate and carbonate have a trigonal planar structure. Attraction assays using nitrate analogs revealed that oxyanions shown as AO3n− (A: N, S, Cl, or C atoms) elicited SCN attraction (Fig. (A)). Oxyanions consist of one atom (N, S, Cl, or C) surrounded by three oxygen atoms in trigonal planar or pyramid structure. Trigonal planar or pyramid structures may need to be recognized in SCN for attraction signals. This idea is supported by the fact that chlorate (trigonal pyramid), which was the most efficient attractant for SCN, is often used as a nitrate analog, to assess the substrate specificity of nitrate (trigonal planar) transporters.Citation24,25) These results indicate the possibility that SCN perceives not only nitrate but also its analogs as chemical attractants. Attraction to nitrate analogs was attenuated by the addition of nitrate to agar media, whereas attraction to azuki root was not affected. (Fig. ) These results indicate that nitrates are not plant-derived attractants, and the mechanism underlying the attraction to nitrate and its analogs is different from that underlying the attraction to plant-derived attractants. Until now, the target proteins of nitrate analogs in SCN attraction are unidentified. One candidate is receptor-type guanylyl cyclase proteins (rGCs), encoded by gcy genes. rGC proteins are required for sensing a number of distinct salt ions in Caenorhabditis elegans.Citation26−28) In addition, three gcy genes have been already identified in SCN.Citation29) In the future, identifying the target protein of nitrate analogs will be useful to control SCN damage.
Nitrates are used as a fertilizer in crop fields worldwide. Our data indicate the possibility that a proper nitrate gradient can disturb SCN behavior. Castro et al. also reported that soil treatment with some salts (NH4NO3, NH4Cl, KNO3, and KCl) reduced the infection of M. incognita J2 in tomato roots.Citation30) However, nitrates can be both an attractant and a repellent at different concentrations. Further studies are needed to establish the method for controlling SCN damage.
Author contribution
S.I. designed the research. Y.S., T.Ko. and S.Y. contributed to the experimental design. A.H. performed and analyzed most of the experiments. T.Ka. performed attraction assay using root tip. S.I. and A.H. wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by JSPS KAKENHI [grant number 16K18695] and Fuji Foundation for Protein Research.
Supplemental data
Supplemental data for this article can be accessed here https://doi.org/10.1080/09168451.2017.1332980.
Supplemental_Material.pptx
Download MS Power Point (345.6 KB)Acknowledgments
We thank Prof. Keiji Tanino (Hokkaido University, Japan) and Dr. Atsuhiko Kushida (NARO Hokkaido Agricultural Research Center, Japan) for kindly providing glycinoeclepin A and SCN-infested soil, respectively.
References
- Wrather JA, Koenning SR. Estimates of disease effects on soybean yields in the United States 2003 to 2005. J Nematol. 2006;38:173–180.
- Wu H, Liu J, Li X, et al. Temporal-spatial population density of Heterodera glycines in soybean roots during the early growth stage. Nematology. 2011;13:79–86.10.1163/138855410X500091
- Kim KS, Vuong TD, Qiu D, et al. Advancements in breeding, genetics, and genomics for resistance to three nematode species in soybean. Theor Appl Genet. 2016;129:2295–2311.10.1007/s00122-016-2816-x
- Liu S, Kandoth PK, Warren SD, et al. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature. 2012;492:256–260.
- Cook DE, Lee TG, Guo X, et al. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science. 2012;338:1206–1209.10.1126/science.1228746
- Concibido VC, Diers BW, Arelli PR. A decade of QTL mapping for cyst nematode resistance in soybean. Crop Sci. 2004;44:1121–1131.10.2135/cropsci2004.1121
- Choe A, von Reuss SH, Kogan D, et al. Ascaroside signaling is widely conserved among nematodes. Curr Biol. 2012;22:772–780.10.1016/j.cub.2012.03.024
- Witzgall P, Kirsch P, Cork A. Sex pheromones and their impact on pest management. J Chem Ecol. 2010;36:80–100.10.1007/s10886-009-9737-y
- Rasmann S, Ali JG, Helder J, et al. Ecology and evolution of soil nematode chemotaxis. J Chem Ecol. 2012;38:615–628.10.1007/s10886-012-0118-6
- Dong L, Li X, Huang L, et al. Lauric acid in crown daisy root exudate potently regulates root-knot nematode chemotaxis and disrupts Mi-flp-18 expression to block infection. J Exp Bot. 2014;65:131–141.10.1093/jxb/ert356
- Dong L, Xu J, Chen S, et al. Mi-flp-18 and Mi-mpk-1 genes are potential targets for Meloidogyne incognita control. J Parasitol. 2016;102:208–213.10.1645/15-768
- Abou-Setta MM, Duncan LW. Attraction of Tylenchulus semipenetrans and Meloidogyne javanica to salts in vitro. Nematropica. 1998;28:49–59.
- Papademetriou MK, Bone LW. Chemotaxis of larval soybean cyst nematode, Heterodera glycines Race 3, to root leachates and ions. J Chem Ecol. 1983;9:387–396.10.1007/BF00988457
- Hida H, Nishiyama H, Sawa S, et al. Chemotaxis assay of plant-parasitic nematodes on a gel-filled microchannel device. Sens Actuators B Chem. 2015;221:1483–1491.10.1016/j.snb.2015.07.081
- Beeman AQ, Njus ZL, Pandey S, et al. Chip technologies for screening chemical and biological agents against plant-parasitic nematodes. Phytopathology. 2016;106:1563–1571.10.1094/PHYTO-06-16-0224-R
- Shiina Y, Tomata Y, Miyashita M, et al. Asymmetric total synthesis of glycinoeclepin A: generateon of a novel bridgehead anion species. Chem Lett. 2010;39:835–837.10.1246/cl.2010.835
- Nonaka S, Katsuyama T, Kondo T, et al. 1,10-Phenanthroline and its derivatives are novel hatching stimulants for soybean cyst nematodes. Bioorg Med Chem Lett. 2016;26:5240–5243.10.1016/j.bmcl.2016.09.052
- Laznik Ž, Trdan S. Attraction behaviors of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) to synthetic volatiles emitted by insect damaged potato tubers. J Chem Ecol. 2016;42:314–322.10.1007/s10886-016-0686-y
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497.10.1111/ppl.1962.15.issue-3
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:151–158.10.1016/0014-4827(68)90403-5
- Becard G, Fortin JA. Early events of vesicular arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988;108:211–218.10.1111/nph.1988.108.issue-2
- Armstrong FAJ, Yohita S, Kamalpreet K. Determination of nitrates in water by ultraviolet spectrophotometry. Anal Chem. 1963;35:1292–1294.
- Hoather RC, Rackham RF. Oxidised nitrogen in waters and sewage effluents observed by ultra-violet spectrophotometry. Analyst. 1959;84:549–551.
- Kinghorn JR, Sloan J, Kana’n GJ, et al. Missense mutations that inactivate the Aspergillus nidulans nrtA gene encoding a high-affinity nitrate transporter. Genetics. 2005;169:1369–1377.
- Akhtar N, Karabika E, Kinghorn JR, et al. High–affinity nitrate/nitrite transporters NrtA and NrtB of Aspergillus nidulans exhibit high specificity and different inhibitor sensitivity. Microbiology. 2015;161:1435–1446.10.1099/mic.0.000088
- Ortiz CO, Faumont S, Takayama J, et al. Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr Biol. 2009;19:996–1004.10.1016/j.cub.2009.05.043
- Smith HK, Luo L, O’Halloran D, et al. Defining specificity determinants of cGMP mediated gustatory sensory transduction in Caenorhabditis elegans. Genetics. 2013;194:885–901.10.1534/genetics.113.152660
- Singhvi A, Liu B, Friedman CJ, et al. A glial K/Cl transporter controls neuronal receptive ending shape by chloride inhibition of an rGC. Cell. 2016;165:936–948.10.1016/j.cell.2016.03.026
- Yan Y, Davis EL. Characterisation of guanylyl cyclase genes in the soybean cyst nematode, Heterodera glycines. Int J Parasitol. 2002;32:65–72.10.1016/S0020-7519(01)00315-0
- Castro CE, McKinney HE, Lux S. Plant protection with inorganic ions. J Nematol. 1991;23:409–413.