Abstract
β-Fructofuranosidases belonging to glycoside hydrolase family (GH) 32 are enzymes that hydrolyze sucrose. Some GH32 enzymes also catalyze transfructosylation to produce fructooligosaccharides. We found that Aspergillus kawachii IFO 4308 β-fructofuranosidase (AkFFase) produces fructooligosaccharides, mainly 1-kestose, from sucrose. We determined the crystal structure of AkFFase. AkFFase is composed of an N-terminal small component, a β-propeller catalytic domain, an α-helical linker, and a C-terminal β-sandwich, similar to other GH32 enzymes. AkFFase forms a dimer, and the dimerization pattern is different from those of other oligomeric GH32 enzymes. The complex structure of AkFFase with fructose unexpectedly showed that fructose binds both subsites −1 and +1, despite the fact that the catalytic residues were not mutated. Fructose at subsite +1 interacts with Ile146 and Glu296 of AkFFase via direct hydrogen bonds.
β-Fructofuranosidase (abbreviated as FFase; EC 3.2.1.26) catalyzes the hydrolysis of sucrose into glucose (Glc) and fructose (Fru). Some FFases also have a strong transfructosylating activity.Citation1) FFases are classified into glycoside hydrolase families (GH) 32 and 68 in the CAZy database.Citation2) Both GH32 enzymes and GH68 enzymes have a five-bladed β-propeller catalytic domain.Citation3) GH32 and GH68 are categorized into clan GH-J in the CAZy database.
Oligosaccharides containing Fru residues are known as fructooligosaccharides (FOS), which promote the growth of beneficial gut bacteria.Citation4) Among the FOS, 1-kestose (β-D-Fruf-(2 → 1)-β-D-Fruf-(2 ↔ 1)-α-D-Glcp) exhibits a strong prebiotic activity.Citation5,6) FOS and related sugars are produced commercially from sucrose using transfructosylation by FFases, and some FFases with strong transfructosylating activity, also called fructosyltransferases, have been identified.Citation1,7) A GH68 FFase from Microbacterium saccharophilum K-1 (MsFFase) catalyzes transfructosylation to produce lactosucrose (β-D-Galp-(1 → 4)-α-D-Glcp-(1 ↔ 2)-β-D-Fruf) from a mixture of sucrose and lactose.Citation8) GH32 FFases from Aspergillus niger ATCC 20611.Citation9) and Aspergillus japonicus CB05 (AjFFase).Citation7,Citation10) produce FOS, mainly 1-kestose and nystose (β-D-Fruf-(2 → 1)-β-D-Fruf-(2 → 1)-β-D-Fruf-(2 ↔ 1)-α-D-Glcp), from sucrose. In contrast, FFases that exhibit a weak ability to produce FOS, such as Aureobasidium pullulans FFase, have been found.Citation11) A combination of alignment and structural analyses of GH32 enzymes indicated that low-level FOS-producing enzymes contain a WMNDPNG motif, while high-level FOS-producing enzymes contain a GQIGDPC motif.Citation11)
Aspergillus kawachii IFO 4308 is a filamentous fungus that is used to brew alcoholic beverages. The whole-genome sequence of A. kawachii IFO 4308 has been determined.Citation12) The genome sequence indicated that A. kawachii possesses a gene for a GH32 FFase (AkFFase). That enzyme has the GQIGDPC motif in its primary structure and shows 68% sequence identity to both AjFFase and A. niger FFase. Here, we show that AkFFase produces FOS consisting mainly of 1-kestose from sucrose, indicating that the enzyme is useful for the production of FOS. We determined the crystal structure of AkFFase, which provided insights into the transfructosylation mechanism of GH32 enzymes.
Materials and methods
Construction of the expression plasmid for AkFFase
A. kawachii IFO 4308 was cultivated as described.Citation13) Total RNA isolation from A. kawachii and first-strand cDNA synthesis were performed by standard procedures as described previously.Citation14) The DNA fragment encoding AkFFase was amplified by PCR using cDNA and primers. The PCR primers were designed using the genome sequence of A. kawachii (DDBJ/EMBL/GenBank ID, DF126461). Using the Signal-P4 server (https://www.cbs.dtu.dk/services/SignalP/),Citation15) the N-terminal signal sequence of AkFFase was predicted to comprise the 24 amino acid residues MKLQTASVLLGSAAAASPSMQTRA. That sequence was omitted in the primer design, and the primers 5′-AGC CAT GAA TTC TCC GTG GTC ATC GAC TAC-3′ and 5′-CTC GAG AAG CTT TCA ATA CTG ACG ATC CGG-3′ (restriction sites of EcoRI and HindIII underlined) were used for PCR amplification. The amplified product was then ligated into the pET-32a(+) vector (Merck Millipore, Darmstadt, Germany). The amplified sequence was verified to be identical to the deduced exon sequence of AkFFase (UniProt ID, G7XM46) by DNA sequencing.
Protein expression and purification
Escherichia coli strain BL21 (DE3) was transformed with the AkFFase expression plasmid. The transformant was grown at 37 °C in 500 mL medium containing 10 mg/mL tryptone, 5 mg/mL yeast extract, 10 mg/mL sodium chloride, and 50 μg/mL ampicillin. When the culture reached an absorbance of 0.6 at 600 nm, protein expression was induced with isopropyl-β-D-thiogalactopyranoside at a final concentration of 0.1 mM, and the culture was incubated for 18 h at 18 °C. The cells were harvested by centrifugation at 4,000 g for 5 min, resuspended in 30 mL 20 mM Tris-HCl buffer (pH 8.0), and then disrupted by sonication. After centrifugation at 12,000 g for 20 min to remove insoluble material, the supernatant was applied onto a nickel nitrilotriacetic acid agarose column (10 mL; QIAGEN, Hilden, Germany) equilibrated with the same buffer. The column was washed with the same buffer, and the enzyme was eluted with 50 mM imidazole in 20 mM Tris-HCl buffer (pH 8.0). The fractions containing the protein were collected, and the purity was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Measurement of the enzymatic activity of tagged AkFFase
The activity for 2% sucrose was measured in 50 mM sodium phosphate buffer (pH 6.0) for 10 min at 30 °C. The reaction (in a 500 μL volume) was stopped by adding 200 μL 0.2 M Na2HPO4–NaOH (pH 11.5), and 5 μL of the solution was taken and mixed with 200 μL Glucose C-II Test Kit solution (Wako Pure Chemical, Osaka, Japan). The absorbance at 505 nm was measured after 5 min of incubation at 37 °C, and the activity was determined with glucose as a standard. One unit (U) of enzymatic activity was defined as the amount of enzyme needed to liberate 1 μmol/min glucose from sucrose. The protein concentration of the purified AkFFase was determined by measuring the absorbance at 280 nm using the molar extinction coefficient (thioredoxin-His-tagged form, 1 mg/mL = 1.710; tag-removed form, 1 mg/mL = 1.851) calculated using the ExPASy ProtParam server (https://web.expasy.org/protparam/).
Thin layer chromatography (TLC)
The purified, tagged AkFFase (10 μg/mL under the reaction condition) was incubated with 30% (w/v) sucrose or fructose in 50 mM Tris-HCl buffer (pH 7.0) at 37 °C for 1 h. Samples withdrawn at different times were immediately heated at 100 °C for 5 min. The samples obtained were then analyzed by TLC on a Silica Gel 60 plate (Merck) using developing solvent with 1-butanol/ethanol/water (5:5:2, v/v/v). The spots were detected by charring with 5% sulfuric acid in methanol.
Determination of the molecular mass of AkFFase by gel filtration
To perform gel filtration, the thioredoxin-His tag of the purified protein was cleaved. Thrombin protease (10 U; Wako Pure Chemical) was added to 10 mg tagged AkFFase in 20 mM Tris-HCl buffer (pH 8.0) and incubated at room temperature for 18 h. The thioredoxin-His tag was then removed by ultrafiltration using an Amicon Ultra-15 Centrifugal Filter Unit 30 K (Merck Millipore). Removal of the tag was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Gel filtration was performed on a Superdex 200 HR 10/30 column (GE Healthcare, Chalfont St. Giles, UK) equilibrated with 10 mM Tris-HCl (pH 7.0) containing 200 mM NaCl using an ÄKTA purifier chromatography system (GE Healthcare) at a flow rate of 0.5 mL/min at room temperature. The protein elution was monitored at 280 nm. The elution was calibrated using size markers, blue dextran (2,000 kDa), thyroglobulin (669 kDa), ferritin (440 kDa), catalase (230 kDa), bovine serum albumin (68 kDa), and ovalbumin (43 kDa).
Crystallization, data collection, and structure determination
The purified, tagged protein was concentrated to 20 mg/mL in 10 mM Tris-HCl (pH 7.0) using an Amicon Ultra-15 Centrifugal Filter Unit 30 K. AkFFase was crystallized at 20 °C using the hanging-drop vapor diffusion method, in which 1.0 μL protein solution was mixed with an equal volume of crystallization reservoir solution containing 2.6 M sodium formate and 0.1 M sodium acetate (pH 5.0, for AkFFase-glycerol and AkFFase-Fru) or 0.1 M sodium phosphate (pH 6.0, for unliganded AkFFase), and crystals with a size of approximately 0.2 mm were obtained. To obtain the unliganded structure, the crystal was cryoprotected with a 2:1 mixture of Paratone-N (Hampton Research, Aliso Viejo, CA, USA) and paraffin oil. To obtain the structures of AkFFase-glycerol and AkFFase-Fru, the crystals were soaked in the reservoir solution supplemented with 35% (w/v) glycerol or 35% (w/v) Fru and then flash-cooled in liquid nitrogen. The solution containing glycerol or fructose also acted as a cryoprotectant. Attempts to soak the crystals in a solution containing glucose, sucrose, 1-kestose, or nystose were unsuccessful. Diffraction data were collected using an AR-NW12A beamline (Photon Factory, Tsukuba, Japan). Data images were processed and scaled using HKL2000.Citation16)
Model building and structure analysis
The structures were solved by the molecular replacement method with MOLREPCitation17) in the CCP4 program suite.Citation18) To solve the unliganded structure, a model of AjFFase (PDB ID, 3LDK) without ligands Citation7) was employed as a probe model. Automated model building was carried out with the ARP/wARP program.Citation19) Refinement was performed using REFMAC5 Citation20) in the CCP4 suite, and manual adjustment and rebuilding of the model were carried out using COOT.Citation21) Validation of the structures was carried out using RAMPAGE Citation22) in the CCP4 suite. The figures were produced using PyMOL (https://www.pymol.org/), Caver,Citation23) and Ligplot+.Citation24) To generate a model of lactosucrose, the coordinates of α-lactose (PDB ID, 1KNM) Citation25) and sucrose (PDB ID, 1YLJ)Citation26) were combined. The coordinate of nystose was obtained from a structure of AjFFase in complex with nystose (PDB ID, 3LEM).Citation7) The data collection and refinement statistics are summarized in Table . The coordinates and structure factors were deposited in the Protein Data Bank under the accession codes 5XH8, 5XH9, and 5XHA.
Table 1. Data collection and refinement statistics.
Results and discussion
Enzymatic properties of recombinant AkFFase
We measured the enzymatic activity of purified thioredoxin-His-tagged AkFFase. The specific activity for 2% sucrose at 30 °C was determined to be 613 ± 17 U/mg (mean ± standard deviation). The specific activity of AjFFase for 2% sucrose has been reported to be 2,286 U/mg, but that measurement was performed at 37 °C.Citation10) We investigated the action of AkFFase for 30% sucrose by TLC, and found that the main product was 1-kestose (Fig. (A)). There was no reaction for 30% fructose (Fig. (B)).
Figure 1. Enzymatic properties of AkFFase.
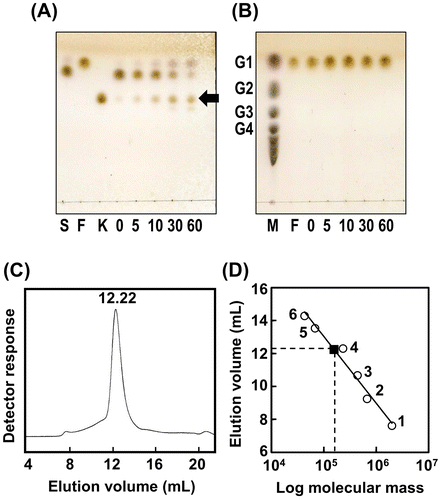
We removed the thioredoxin-His tag from the recombinant protein and analyzed the molecular mass of AkFFase by gel filtration (Fig. (C) and (D)). The molecular mass of the monomer protein, calculated based on the amino acid sequence, was 70 kDa. Comparison of the elution volume of AkFFase with those of size markers indicated that the molecular mass was 157 kDa, suggesting that AkFFase forms a dimer in solution. AjFFase has also been observed as a dimer on gel filtration,Citation10) and A. niger FFase has been found as an oligomer.Citation9)
Structure determination and overall structure of unliganded AkFFase
We expressed recombinant AkFFase fused to a thioredoxin-His tag in Escherichia coli and affinity-purified the protein by Ni-NTA-agarose chromatography. We determined the crystal structure of the unliganded AkFFase and the AkFFase-glycerol complex at 2.3-Å and 2.1-Å resolutions, respectively (Table ). The crystal belongs to the trigonal space group P3121, with one monomer in the asymmetric unit. The 2|Fo| − |Fc| electron density contoured at 1 σ showed continuous density for almost all the atoms of AkFFase, but the N-terminal portion of the thioredoxin-His tag was not visible. Although we did not add thrombin protease to the protein solution for the crystallization, it is likely that the N-terminal portion was cleaved by some proteases from E. coli during the purification procedure, and the cleaved portion (about 20 kDa) was then removed by the ultrafiltration step. A Ramachandran plot calculated using RAMPAGECitation22) identified one residue, Asn571, as an outlier, although the electron density was well defined.
AkFFase is composed of four regions, an N-terminal small component (residues 25–59; component NS), a β-propeller domain (residues 60–440), an α-helical linker (residues 441–452), and a C-terminal β-sandwich domain (residues 453–628; Fig. (A)). The combination of the β-propeller catalytic domain and the β-sandwich domain is the hallmark of GH32 enzymes, and the β-propeller fold of GH32 is composed of five blades, designated I–V (Fig. (B)). A sodium ion appears to be present between blade IV and blade V of AkFFase, because refinement with other metal atoms produced a strong negative |Fo| − |Fc| map. The binding of sodium ion was probably due to the high concentration (2.6 M) of sodium formate used for the crystallization and thus is likely to be an artifact.
Figure 2. Overall structure of AkFFase.
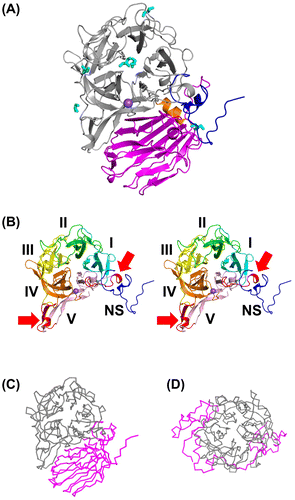
We carried out a structural similarity search using the DALI server.Citation27) As documented previously,Citation1,Citation7) the N-terminal β-propeller domain is homologous to other proteins containing a five-bladed β-propeller domain; including GH43, GH62, GH68, GH117, and GH130,Citation28,29) and the C-terminal domain is similar to various lectins or lectin-like proteins (Table ). Comparison of GH32 AkFFase (Fig. (C)) with GH68 MsFFase (Fig. (D)) indicated that the structures other than the β-propeller domain are highly dissimilar despite the fact that the proteins are categorized into the same clan, GH-J.
Table 2. Summary of structural similarity search using the DALI server.
The structure of AkFFase-glycerol was virtually isomorphous to that of the unliganded AkFFase, and there was no significant structural difference between the unliganded AkFFase and AkFFase-glycerol. We identified six glycerol molecules in AkFFase-glycerol; two appeared in the active site, while the other four appeared on the protein surface of the N-terminal domain (Fig. (A)). GH32 enzymes are retaining glycosidases, and two Asp residues and one Glu residue form a catalytic triad, which is located at the center of the β-propeller domain.Citation3) Comparison with other GH32 enzymes indicated that Asp64, Asp194, and Glu271 function as a catalytic nucleophile, a transition-state stabilizer, and a general acid/base catalyst, respectively. Based on the positions of the catalytic residues, we determined that the two glycerol molecules found in the active site were located at subsites −1 and +1, respectively. Glycerol at subsite −1 forms three direct hydrogen bonds with AkFFase (O1-Asp194OD1, O1-Asp122 N, and O2-Asp64OD2), and glycerol at subsite +1 forms a direct hydrogen bond with AkFFase (O1-Ile146O). The four glycerol molecules on the protein surface are likely to be artifacts, as they interact with only a few amino acid residues of AkFFase.
Component NS and some loop regions distantly located from the catalytic center
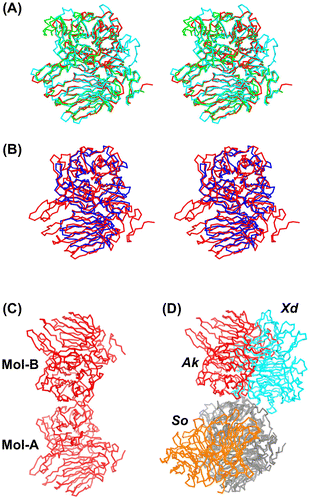
We compared the Cα backbones of some GH32 enzymes. AkFFase is highly similar to fungal enzymes (AjFFaseCitation7) and Xanthophyllomyces dendrorhous FFase (XdFFaseCitation30)), but some loop regions located far from the active site are not conserved (Fig. (A)). When compared with a bacterial FFase from Thermotoga maritima,Citation31) AkFFase is characterized by the presence of component NS and a larger-sized blade V (Fig. (B)).
Figure 3. Comparison of the Cα backbones of AkFFase and related GH32 enzymes.
The structure of component NS contains no α-helices or β-sheet besides one short 3–10 helix (residues 37–40). Except for some N-termini of GH32 enzymes, there was no obvious similarity to other proteins in the DALI search. In blade V of AkFFase, two extra regions, residues 374–388 and 407–428, are extended from the blade (Fig. (B)). Residues in component NS interact with the extra regions of blade V via six hydrogen bonds (Ser38O-Gln386NE2, Leu40O-Gln386NE2, Gly43 N-Asn385O, Gly43 N-Gln386O, Ser44 N-Gln386O, and Leu45 N-Gln386O). Generally, the β-strands in blades I and V of the five-bladed β-propeller folds form many hydrogen bonds to produce a so-called “molecular Velcro” structure.Citation29,32) It is likely that the hydrogen bonds generated by component NS and the extra regions of blade V have a function similar to the molecular Velcro found in other five-bladed β-propeller folds.
Trollope et al. introduced mutations in some loop regions of AjFFase, and found that a four-substitution variant; Phe140 → Tyr, Ala178 → Pro, Gly321 → Asn, and Gln490 → Ser; resulted in improved FOS production.Citation33) It should be noted that the four corresponding residues in AkFFase are Tyr143, Pro181, Asp299, and Asn463, indicating that the four residues are not conserved between AkFFase and AjFFase, and that two out of the four residues in AkFFase are identical to those in the four-substitution variant AjFFase. It is, however, difficult to evaluate whether the loop regions located distantly from the catalytic center contribute to the enzymatic properties of the protein based on the structural snapshot of AkFFase.
Dimer structure and a tunnel at the bottom of the catalytic cleft
Gel filtration analysis indicated that AkFFase is a dimer in solution (Fig. (C) and (D)). Analysis of molecular interactions calculated using the PISA server Citation34) revealed that AkFFase forms a dimer (Fig. (C)). The dimeric structure forms via interaction between the two β-propeller domains. The fungal FFases XdFFase Citation30) and Schwanniomyces occidentalis FFase (SoFFase) Citation35) have been reported to form dimers. To compare the dimer formations of AkFFase, XdFFase, and SoFFase, we superimposed one of the subunits (Fig. (D)). Despite the similarity of the subunit structures, the positions of the counterpart subunits were completely different, while the contact areas of the dimers of AkFFase, XdFFase, and SoFFase showed similar values, 6.2 × 103, 7.2 × 103, and 5,4 × 103 Å2, respectively. The dimer structure of AkFFase looks atypical, but the importance of the dimer formation remains uncertain. A GH68 levansucrase from Zymomonas mobils has been reported to form an oligomeric structure at pH values below 6.0 and mainly produces levan under acidic conditions.Citation36) The dimer structure of XdFFase has also been considered as an architecture that accommodates a polymer substrate.Citation30) The spatial relationship between the two subunits of AkFFase is different than that of XdFFase, however, so that explanation could not be applied to the case of AkFFase.
Another conspicuous feature of AkFFase is a tunnel at the bottom of the catalytic cleft. We found numerous water molecules in the tunnel (Fig. (A)). A similar tunnel has been observed in GH68 MsFFase.Citation37) Some other carbohydrate hydrolases have also been reported to have a water path or a buried water channel near the active site.Citation38–41) Although the precise role of the water path is not clear, it is proposed to function as a water reservoir and/or a water drain.
Figure 4. Structure of the AkFFase-Fru complex.
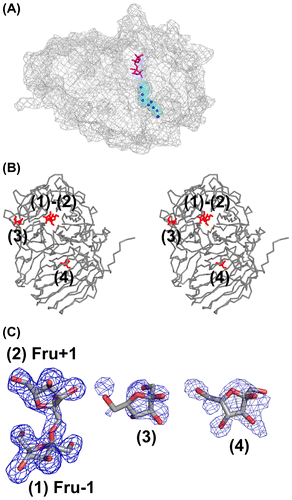
Fru-bound structure
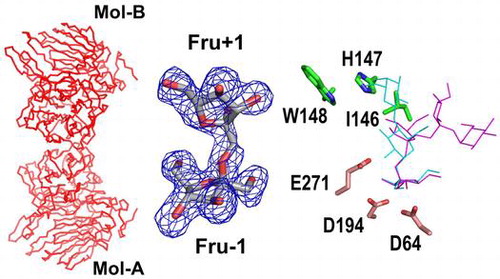
We determined the complex structure of AkFFase with Fru (AkFFase-Fru) at 2.1-Å resolution. The structure was almost isomorphous to that of the unliganded AkFFase, and there was no significant structural difference between AkFFase-glycerol and AkFFase-Fru. There are four Fru molecules in the structure of AkFFase-Fru; two molecules (indicated as (1) and (2) in Fig. (B)) appeared in the catalytic cleft, and the other two molecules (indicated as (3) and (4) in Fig. (B)) appeared on the surface of AkFFase. We identified the two molecules in the catalytic cleft as a continuous electron density map (Fig. (C)), which can be modeled as a disaccharide (Fru-β-(2 → 1)-Fru) molecule at subsites −1 and +1 (labeled as Fru −1 and Fru +1). Although several structures of wild-type GH32 and GH68 enzymes in complex with Fru have been reported (PDB IDs: 3KF3,Citation35) 3VSS,Citation37) 3PIJ,Citation42) 1Y9G,Citation43) 2ADE,Citation44) and 2XQR Citation45)), the case of AkFFase is, to our knowledge, the first observation that Fru appears at subsite +1 without any mutations in the catalytic residues. That result indicated that subsite +1 of AkFFase has a strong affinity for Fru. The electron density for the other two Fru molecules ((3) and (4) in Fig. (C)) was weak and better resolved at the lower-contoured level. Hence, it is likely that those two Fru molecules are artifacts.
We tested whether AkFFase catalyzes the condensation of Fru to produce the disaccharide. We incubated a high concentration (30%) of Fru with AkFFase and analyzed the reaction solution by TLC, but we did not observe a condensation reaction (Fig. (B)). Therefore, it remains unknown whether the two Fru molecules were independently present at both subsites −1 and +1 or whether Fru-β-(2 → 1)-Fru could be produced in the crystal packing environment. We analyzed the interaction between AkFFase and the two Fru molecules in the catalytic cleft using Ligplot+Citation24) (Fig. ). Fru −1 forms hydrogen bonds directly with five amino acid residues: Asp64, Asp122, Arg193, Asp194, and Glu271. In contrast, Fru +1 forms two direct hydrogen bonds with amino acid residues Ile146 and Glu296 (O4-Ile146O and O3-Glu296OE1), while other hydrogen bonds between Fru + 1 and amino acid residues are formed via water molecules. Atoms O2 and O5 of Fru +1 do not form hydrogen bonds with any amino acid residues.
Figure 5. Schematic drawing of the amino acid residues interacting with Fru −1 and Fru +1.
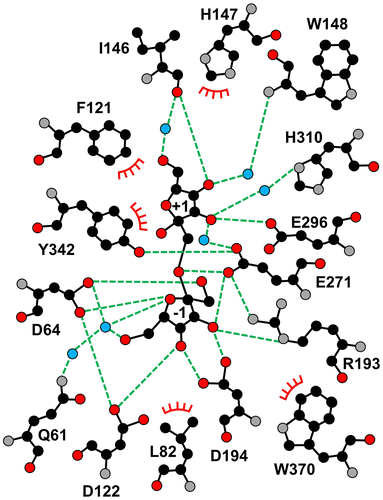
The amino acid residues interacting with Fru −1 and Fru +1 and the corresponding residues in some GH32 and GH68 enzymes are listed in Table . The residues in subsite −1 are relatively conserved regardless of whether the enzyme is GH32 or GH68, and the consensus sequences of subsite −1 are identified in Table . On the other hand, subsite +1 contains a wide variety of residues, except for one residue equivalent to Tyr342 of AkFFase. In particular, AkFFase contains three successive residues (Ile146-His147-Trp148) located in an extended loop connecting two β-strands in blade II, while most GH32 and GH68 enzymes do not possess corresponding residues.
Table 3. Amino acid residues in the catalytic cleft of AkFFase and the corresponding residues in other clan GH-J enzymes.
Insights into the transfructosylation of AkFFase
The FFases from Aspergillus species have been reported to possess a strong ability to synthesize FOS, and the GQIGDPC motif is responsible for product specificity.Citation11) However, the yeast FFases such as SoFFase has also transfructosylation activity,Citation46) and a mutagenesis study of two underlined residues in the WMNDPNG motif (Trp47 and Asn49 in SoFFase) suggested that the motif is involved in hydrolase/transferase activity.Citation11,47) In addition, two Trp residues (Trp76 and Trp314 in SoFFase) form the catalytic cleft of yeast FFases along with the Asn and Trp residues (Fig. (A)). These four amino acid residues are not conserved in AkFFase, and the transfructosylation mechanism appears to be different between the Aspergillus FFases and the yeast FFases. The dimer formation of SoFFase produces a unique catalytic cleft that plays important role in the substrate specificity of the enzyme.
Figure 6. Structure of the catalytic cleft of AkFFase.
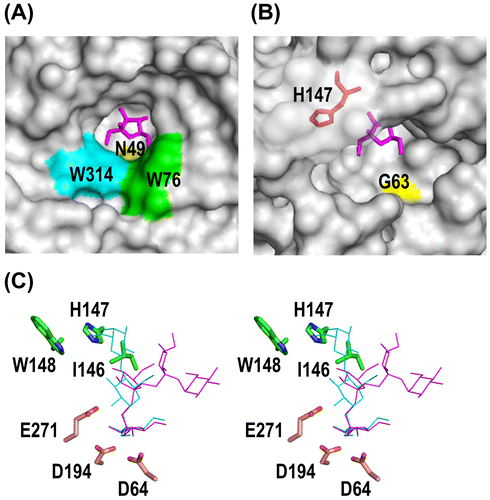
In AkFFase, the corresponding residues of Trp47 and Asn49 in SoFFase are identified as Gln61 and Gly63. Gln61 forms a hydrogen bond with Fru −1 via two water molecules (Fig. ), but the distance between atom NE2 of Gln61 and atom O6 of Fru −1 is large at 8.4 Å. The distance between atom N of Gly63 and atom O6 of Fru -1 is also large at 7.3 Å (Fig. (B)). Those findings suggest that the interactions of the two residues with the substrate are weak. In addition, no amino acid residues equivalent to Trp76 and Trp314 of SoFFase are identified in AkFFase. The surface model shown in Fig. (B) indicated that the catalytic cleft of AkFFase appears to allow another sucrose molecule to enter into the active site during catalysis. Fig. (B) also shows that in AkFFase, a loop containing His147 covers half of the catalytic cleft. The IHW sequence in subsite + 1 of AkFFase appears to accommodate the Fru + 1 residue, which may result in the high level of FOS synthesis.
Some GH68 enzymes have an ability to produce lactosucrose, a trisaccharide, from a mixture of sucrose and lactose.Citation37,48,49) To determine whether AkFFase produces lactosucrose, we placed models of lactosucrose and nystose in the structure of AkFFase (Fig. (C)). When we placed the model of lactosucrose on the catalytic cleft of AkFFase, the simulation predicted a steric crash between His147 of the IHW sequence in AkFFase and the galactose residue in lactosucrose. That suggested that AkFFase is not suitable for producing lactosucrose. In contrast, the model of nystose fit well on the catalytic cleft of AkFFase, indicating that AkFFase is an ideal enzyme to produce FOS.
Funding
This work was supported in part by a Grant-in-Aid to T. Tonozuka [grant number 16K07687] from the Japan Society for the Promotion of Science.
Disclosure statement
No potential conflict of interest was reported by the authors.
Author’s contribution
T. Tochio, K. H., and T. Tonozuka planned the experiments. T. Tochio prepared the cDNA. M. K. and S. S. constructed the expression plasmid. M. N. and Y. G. crystallized the enzyme and collected the X-ray diffraction data. M. N. performed TLC and gel filtration. T. Tonozuka and M. N. determined the structure and wrote the manuscript. T. Tonozuka and A. N. edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was performed with the approval of the Photon Factory Program Advisory Committee (2016G013).
Notes
Abbreviations: Ak, Aspergillus kawachii; Aj, Aspergillus japonicus; FFase, β-fructofuranosidase; FOS, fructooligosaccharides; GH, glycoside hydrolase family; Ms, Microbacterium saccharophilum; TLC, thin layer chromatography; So, Schwanniomyces occidentalis; Xd, Xanthophyllomyces dendrorhous.
References
- Velázquez-Hernández ML, Baizabal-Aguirre VM, Bravo-Patiño A, Cajero-Juárez M, Chávez-Moctezuma MP, Valdez-Alarcón JJ. Microbial fructosyltransferases and the role of fructans. J Appl Microbiol. 2009;106:1763–1778.10.1111/jam.2009.106.issue-6
- Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495.10.1093/nar/gkt1178
- Lammens W, Le Roy K, Schroeven L, Van Laere A, Rabijns A, Van den Ende W. Structural insights into glycoside hydrolase family 32 and 68 enzymes: functional implications. J Exp Bot. 2009;60:727–740.10.1093/jxb/ern333
- Bali V, Panesar PS, Bera MB, Panesar R. Fructo-oligosaccharides: production, purification and potential applications. Crit Rev Food Sci Nutr. 2015;55:1475–1490.10.1080/10408398.2012.694084
- Koga Y, Tokunaga S, Nagano J, Sato F, Konishi K, Tochio T, Murakami Y, Masumoto N, Tezuka J, Sudo N, Kubo C, Shibata R. Age-associated effect of kestose on Faecalibacterium prausnitzii and symptoms in the atopic dermatitis infants. Pediatr Res. 2016;80:844–851.10.1038/pr.2016.167
- Tochio T, Kitaura Y, Nakamura S, Sugawa C, Takahashi M, Endo A, Shimomura Y. An alteration in the cecal microbiota composition by feeding of 1-kestose results in a marked increase in the cecal butyrate content in rats. PLoS One. 2016;11:e0166850.10.1371/journal.pone.0166850
- Chuankhayan P, Hsieh CY, Huang YC, Hsieh YY, Guan HH, Hsieh YC, Tien YC, Chen CD, Chiang CM, Chen CJ. Crystal structures of Aspergillus japonicus fructosyltransferase complex with donor/acceptor substrates reveal complete subsites in the active site for catalysis. J Biol Chem. 2010;285:23251–23264.10.1074/jbc.M110.113027
- Ohta Y, Hatada Y, Hidaka Y, Shimane Y, Usui K, Ito T, Fujita K, Yokoi G, Mori M, Sato S, Miyazaki T, Nishikawa A, Tonozuka T. Enhancing thermostability and the structural characterization of Microbacterium saccharophilum K-1 β-fructofuranosidase. Appl Microbiol Biotechnol. 2014;98:6667–6677.10.1007/s00253-014-5645-3
- Hirayama M, Sumi N, Hidaka H. Purification and properties of a fructooligosaccharide-producing β-fructofuranosidase from Aspergillus niger ATCC 20611. Agric Biol Chem. 1989;53:667–673.
- Duan KJ, Sheu DC, Chen JS. Purification and characterization of β -fructofuranosidase from Aspergillus japonicus TIT-KJ1. Biosci Biotechnol Biochem. 1993;57:1811–1815.10.1271/bbb.57.1811
- Trollope KM, van Wyk N, Kotjomela MA, Volschenk H. Sequence and structure-based prediction of fructosyltransferase activity for functional subclassification of fungal GH32 enzymes. FEBS J. 2015;282:4782–4796.10.1111/febs.13536
- Futagami T, Mori K, Yamashita A, Wada S, Kajiwara Y, Takashita H, Omori T, Takegawa K, Tashiro K, Kuhara S. Goto M: Genome sequence of the white koji mold Aspergillus kawachii IFO 4308, used for brewing the Japanese distilled spirit shochu. Eukaryot Cell. 2011;10:1586–1587.10.1128/EC.05224-11
- Ito K, Ogasawara H, Sugimoto T, Ishikawa T. Purification and properties of acid stable xylanases from Aspergillus kawachii. Biosci Biotechnol Biochem. 1992;56:547–550.10.1271/bbb.56.547
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2001.
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786.10.1038/nmeth.1701
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326.10.1016/S0076-6879(97)76066-X
- Vagin A, Teplyakov A. Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr. 2010;66:22–25.10.1107/S0907444909042589
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–242.10.1107/S0907444910045749
- Langer GG, Hazledine S, Wiegels T, Carolan C, Lamzin VS. Visual automated macromolecular model building. Acta Crystallogr D Biol Crystallogr. 2013;69:635–641.10.1107/S0907444913000565
- Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA. REFMAC5 for the refinement of macromolecular crystal structures. Acta Cryst D Biol Crystallogr. 2011;67:355–367.10.1107/S0907444911001314
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501.10.1107/S0907444910007493
- Lovell SC, Davis IW, Arendall WB 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Cα geometry: φ, ψ, and Cβ deviation. Proteins. 2003;50:437–450.10.1002/prot.10286
- Chovancova E, Pavelka A, Benes P, Strnad O, Brezovsky J, Kozlikova B, Gora A, Sustr V, Klvana M, Medek P, Biedermannova L, Sochor J, Damborsky J. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput Biol. 2012;8:e1002708.10.1371/journal.pcbi.1002708
- Laskowski RA, Swindells MB. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786.10.1021/ci200227u
- Notenboom V, Boraston AB, Williams SJ, Kilburn DG, Rose DR. High-resolution crystal structures of the lectin-like xylan binding domain from Streptomyces lividans xylanase 10A with bound substrates reveal a novel mode of xylan binding. Biochemistry. 2002;41:4246–4254.10.1021/bi015865j
- Chen Y, Delmas J, Sirot J, Shoichet B, Bonnet R. Atomic resolution structures of CTX-M β-lactamases: extended spectrum activities from increased mobility and decreased stability. J Mol Biol. 2005;348:349–362.10.1016/j.jmb.2005.02.010
- Holm L, Rosenström P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–W549.10.1093/nar/gkq366
- Pons T, Naumoff DG, Martínez-Fleites C, Hernández L. Three acidic residues are at the active site of a β-propeller architecture in glycoside hydrolase families 32, 43, 62, and 68. Proteins. 2004;54:424–432.
- Tonozuka T, Tanaka Y, Okuyama S, Miyazaki T, Nishikawa A, Yoshida M. Structure of the catalytic domain of α-l-arabinofuranosidase from Coprinopsis cinerea, CcAbf62A, provides insights into structure–function relationships in glycoside hydrolase family 62. Appl Biochem Biotechnol. 2017;181:511–525.10.1007/s12010-016-2227-0
- Ramírez-Escudero M, Gimeno-Pérez M, González B, Linde D, Merdzo Z, Fernández-Lobato M, Sanz-Aparicio J. Structural analysis of β-fructofuranosidase from Xanthophyllomyces dendrorhous reveals unique features and the crucial role of N-glycosylation in oligomerization and activity. J Biol Chem. 2016;291:6843–6857.10.1074/jbc.M115.708495
- Alberto F, Bignon C, Sulzenbacher G, Henrissat B, Czjzek M. The three-dimensional structure of invertase (β-fructosidase) from Thermotoga maritima reveals a bimodular arrangement and an evolutionary relationship between retaining and inverting glycosidases. J Biol Chem. 2004;279:18903–18910.10.1074/jbc.M313911200
- Fülöp V, Jones DT. β Propellers: structural rigidity and functional diversity. Curr Opin Struct Biol. 1999;9: 715–721.10.1016/S0959-440X(99)00035-4
- Trollope KM, Görgens JF, Volschenk H. Semirational directed evolution of loop regions in Aspergillus japonicus β-fructofuranosidase for improved fructooligosaccharide production. Appl Environ Microbiol. 2015;81:7319–7329.10.1128/AEM.02134-15
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797.10.1016/j.jmb.2007.05.022
- Álvaro-Benito M, Polo A, González B, Fernández-Lobato M, Sanz-Aparicio J. Structural and kinetic analysis of Schwanniomyces occidentalis invertase reveals a new oligomerization pattern and the role of its supplementary domain in substrate binding. J Biol Chem. 2010;285:13930–13941.10.1074/jbc.M109.095430
- Goldman D, Lavid N, Schwartz A, Shoham G, Danino D, Shoham Y. Two active forms of Zymomonas mobilis levansucrase. An ordered microfibril structure of the enzyme promotes levan polymerization. J Biol Chem. 2008;283:32209–32217.10.1074/jbc.M805985200
- Tonozuka T, Tamaki A, Yokoi G, Miyazaki T, Ichikawa M, Nishikawa A, Ohta Y, Hidaka Y, Katayama K, Hatada Y, Ito T, Fujita K. Crystal structure of a lactosucrose-producing enzyme, Arthrobacter sp. K-1 β-fructofuranosidase. Enzyme Microb Technol. 2012;51:359–365.10.1016/j.enzmictec.2012.08.004
- Hondoh H, Saburi W, Mori H, Okuyama M, Nakada T, Matsuura Y, Kimura A. Substrate recognition mechanism of α-1,6-glucosidic linkage hydrolyzing enzyme, dextran glucosidase from Streptococcus mutans. J Mol Biol. 2008;378:913–922.10.1016/j.jmb.2008.03.016
- Chen M, Kostylev M, Bomble YJ, Crowley MF, Himmel ME, Wilson DB, Brady JW. Experimental and modeling studies of an unusual water-filled pore structure with possible mechanistic implications in family 48 cellulases. J Phys Chem B. 2014;118:2306–2315.10.1021/jp408767j
- Okazawa Y, Miyazaki T, Yokoi G, Ishizaki Y, Nishikawa A, Tonozuka T. Crystal structure and mutational analysis of isomalto-dextranase, a member of glycoside hydrolase family 27. J Biol Chem. 2015;290:26339–26349.10.1074/jbc.M115.680942
- Teze D, Hendrickx J, Dion M, Tellier C, Woods VL Jr, Tran V, Sanejouand YH. Conserved water molecules in family 1 glycosidases: a dxms and molecular dynamics study. Biochemistry. 2013;52:5900–5910.10.1021/bi400260b
- Bujacz A, Jedrzejczak-Krzepkowska M, Bielecki S, Redzynia I, Bujacz G. Crystal structures of the apo form of β-fructofuranosidase from Bifidobacterium longum and its complex with fructose. FEBS J. 2011;278:1728–1744.10.1111/j.1742-4658.2011.08098.x
- Nagem RAP, Rojas AL, Golubev AM, Korneeva OS, Eneyskaya EV, Kulminskaya AA, Neustroev KN, Polikarpov I. Crystal structure of exo-inulinase from Aspergillus awamori: the enzyme fold and structural determinants of substrate recognition. J Mol Biol. 2004;344:471–480.10.1016/j.jmb.2004.09.024
- Verhaest M, Lammens W, Le Roy K, De Ranter CJ, Van Laere A, Rabijns A, Van den Ende W. Insights into the fine architecture of the active site of chicory fructan 1-exohydrolase: 1-kestose as substrate vs sucrose as inhibitor. New Phytol. 2007;174:90–100.10.1111/j.1469-8137.2007.01988.x
- Hothorn M, Van den Ende W, Lammens W, Rybin V, Scheffzek K. Structural insights into the pH-controlled targeting of plant cell-wall invertase by a specific inhibitor protein. Proc Natl Acad Sci USA. 2010;107:17427–17432.10.1073/pnas.1004481107
- Álvaro-Benito M, de Abreu M, Fernández-Arrojo L, Plou FJ, Jiménez-Barbero J, Ballesteros A, Polaina J, Fernández-Lobato M. Characterization of a β-fructofuranosidase from Schwanniomyces occidentalis with transfructosylating activity yielding the prebiotic 6-kestose. J Biotechnol. 2007;132:75–81.10.1016/j.jbiotec.2007.07.939
- Lafraya A, Sanz-Aparicio J, Polaina J, Marin-Navarro J. Fructo-oligosaccharide synthesis by mutant versions of Saccharomyces cerevisiae invertase. Appl Environ Microbiol. 2011;77:6148–6157.10.1128/AEM.05032-11
- Silvério SC, Macedo EA, Teixeira JA, Rodrigues LR. Perspectives on the biotechnological production and potential applications of lactosucrose: a review. J Funct Foods. 2015;19:74–90.10.1016/j.jff.2015.09.014
- Li W, Yu S, Zhang T, Jiang B, Mu W. Recent novel applications of levansucrases. Appl Microbiol Biotechnol. 2015;99:6959–6969.10.1007/s00253-015-6797-5
