Abstract
On extended screening of squalene-producing strains in Okinawa mangrove water, we identified 14 novel squalene-producing thraustochytrids from 172 unialgal clonal isolates. The novel thraustochytrids produced 13.9–7.54 mg squalene/g dry cell weight. Eight isolates were found to belong to potentially novel squalene-producing genera, forming a monophyletic cluster independent from any known thraustochytrids.
Key words:
Thraustochytrids (Labyrinthulomycetes), heterotrophic stramenopiles, are an important part of the marine microbiota as saprotrophic decomposers. They excrete enzymes extracellularly, and recycle nutrients in marine ecosystems.Citation1) Some thraustochytrids are known as microbial resources for polyunsaturated fatty acidsCitation2), eicosapentaenoic acid (EPA)Citation3), docosahexaenoic acid (DHA)Citation4), docosapentaenoic acid, and squalene (SQ).Citation5) EPA, DHA, and SQ have been used in dietary supplements.Citation6,7)
SQ has attracted attention for its biological abilitiesCitation8); it protects cells from oxidative stress, lowers cholesterol levels in serum, and inhibits chemically-induced tumors.Citation9,10) In addition, SQ can be converted into alternative fuels equivalent to gasoline and jet fuel.Citation11) To date, the major commercial sources of SQ are liver oil from deep-sea sharksCitation12) and plant oils.Citation13) However, an increased risk of shark extinction due to their endangered statusCitation14) and a fluctuating supply from plants caused by regional and seasonal variationCitation15) have raised questions about the sustainability of the supply. Therefore, it is important to identify alternative SQ-producing organisms.
Thraustochytrids are saprotrophic decomposers; therefore, it is expected that they utilize waste water as a carbon source for growth. These features suggest that Thraustochytrid sp. can be used to develop a terpenoid-producing host platform, which can result in the production of valuable compounds from scratch as well as a solution for environmental pollution. Aurantiochytrium sp. 18W-13a, a thraustochytrid strain, has been reported to produce high amount of SQ.Citation5,16) In order to expand available resources of SQ-producing strains, 172 unialgal clonal isolates of thraustochytrids were obtained from a mangrove area in Okinawa Prefecture, Japan. The thraustochytrids were isolated by zoospore-inducing methods using the pine pollen-baiting technique. All isolates were confirmed as thraustochytrids based on microscopic observation of morphological features such as spherical to oblong vegetative cells, presence of ectoplasmic nets, and heterokont bi-flagellated zoospores. These isolates were cultured in 40 mL of GPY medium containing 2% glucose, 1% peptone, 0.5% yeast extract, and 50% marine art SF-1 artificial sea water (Tomita Pharmaceutical Co., Ltd., Tokushima, Japan) in 100-mL Erlenmeyer flasks, which were placed in a temperature-controlled reciprocal shaker (25 °C, 100 strokes per min). After culturing for 4–5 days, cells were harvested by centrifugation at 4000 rpm (25 °C for 15 min), and were lyophilized for SQ quantification. A total of 50 mg of lyophilized biomass was suspended in 2 mL of 50% acetone water in a 5-mL glass vial. Suspensions were kept at 25 °C overnight, and then sonicated (UD-200; TOMY, Tokyo, Japan). Homogenized samples were extracted three times with 2 mL of n-hexane. The extract was dried under a gentle N2 flow, re-dissolved in 2 mL of n-hexane, and applied to a silica gel column (0.5 × 3 cm). The pass through was collected, and SQ was eluted with 5 mL of n-hexane. The SQ fraction was dried under a gentle N2 flow, and dissolved in 1 mL of n-hexane. The sample was analyzed by an Agilent 7890 GC/5975 MSD equipped with a 30 m × 250 μm × 0.25 μm HP-5 ms UI column (Agilent, Santa Clara, CA, USA). Helium was used as the carrier gas. One microliter of the sample was injected into a gas chromatography/mass spectrometry (GC/MS) system in split-less mode at a front inlet temperature of 260 °C, initial oven temperature of 120 °C for 1 min, followed by an increase of 10 °C/min to 260 °C, and held for 10 min. The authentic standard of SQ (Sigma Aldrich, St. Louis, MO, USA) was used for quantification and identification.
Among the 172 isolates tested, 132 isolates produced more than 0.1 mg SQ/g dry cell weight, 33 strains produced very little SQ (less than 0.1 mg/g dry cell weight), and the remaining seven did not produce any at all. Biomass of the cultured cells also varied among the isolates, ranging from 1.82 to 23.1 g/L culture. Table shows the top 14 isolates in terms of SQ content. The biomass of the strains showed a wide variation ranging from 2.36 to 7.40 g/L culture. In addition, the strains showed a variation in cell color (from pale orange to orange). These strains produced a minimum of 7.54 mg SQ/g dry cell weight, and the production ranged from 23.1 to 65.2 mg/L culture. Among these, MST1268 showed the highest SQ accumulation (13.9 mg SQ/g dry cell weight). However, the strain produced lesser amounts of SQ than Aurantiochytrium sp. 18W-13aCitation5,16) (173.7–198 mg SQ/g dry cell weight).
Table 1. GC-MS quantification of squalene produced by selected strains.
All obtained clones showed less development of ectoplasmic net, and culture of 14 SQ-producing isolates showed pale orange to orange color, suggesting Aurantiochytrium-like morphological features. The 14 SQ-producing isolates were subjected to molecular phylogenetic analysis to determine the taxonomic classification. The full length 18S rRNA gene sequence was directly amplified using polymerase chain reaction from cultured cells using the amplification primers SR01Citation17) and SR12L1.Citation18) A one pass sequence from the 5′ end of the 18S rRNA gene was determined using the primer SR01 by Sanger sequencing. The determined sequences were aligned with a known sequence data-set based on previous researchCitation18) using ClustalX 2.1Citation19), and then refined manually. Phylogenetic analysis was performed using MEGA 6.0.6.Citation20) The phylogenetic tree was generated by the maximum likelihood (ML) method using the “distances of the Tamura–Nei model” as a substitution model, and the “pairwise deletion” option for the gap treatment.Citation20) Bootstrap values were obtained from 500 re-samplings. These sequences were registered in GenBank under accession numbers LC228934-LC228947.
As shown in Fig. , the topology of the phylogenetic tree was similar to that constructed in previous research.Citation18) In the overall tree, each genus has been reconstructed as an independent cluster. The genus Aurantiochytrium formed a cluster (Aurantiochytrium + UT cluster” in Fig. ) together with the “UT3a, UT3b, UT3c cluster,” as reported in previous research.Citation18) Within this cluster, the “Aurantiochytrium sp. 1 cluster,” the “Au2 cluster,” and the “Aurantiochytrium limacium cluster” were separately branched. Six of the 14 SQ-producing strains (MST3336, MST2684, MST3194, MST2758, MST2692, and MST2894) were allocated inside the “Aurantiochytrium sp. 1 cluster.” The “Aurantiochytrium sp. 1 cluster” also included a strain producing a high level of SQ (Aurantiochytrium sp.18W-13a) and was well supported by the bootstrap probability. These results suggested that these six isolates are a closely-related taxon of Aurantiochytrium sp. 1 producing SQ.
Fig. 1. Maximum likelihood phylogeny of the thraustochytrid based on 18S rRNA sequences.
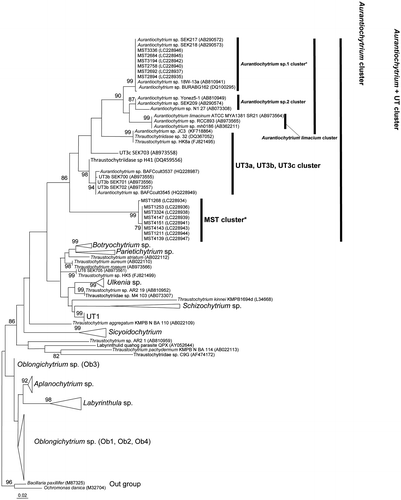
The remaining eight of the top 14 SQ-producing isolates were established as an independent monophyletic cluster (“MST cluster” in Fig. ) that branched out from the root of the “Aurantiochytrium cluster” and the “UT3a, UT3b, UT3c cluster.” Although this independent cluster formed a monophyletic cluster with the “Aurantiochytrium cluster” and “UT3a, UT3b, UT3c cluster,” this cluster was topologically independent from any of those cluster members. This suggested that “MST cluster” members are novel SQ-producing thraustochytrids, although they are closely related to Aurantiochytrium. We also note that the highest SQ producer among these 14 strains was allocated inside this “MST cluster.”
Thus, we found novel thraustochytrid members that are potential SQ producers, the “MST cluster” member isolates. Further screening of the members of this novel cluster, and optimization of the method for cultivating them could provide novel candidates for the commercial production of SQ.
Author contributions
M.O. and S.M designed the experiments. M.O. and A.K. performed experiments. All authors discussed the data. M.O., A.K., and S.T. wrote the paper.
Funding
This work was supported by the RIKEN Competitive Program for Creative Science and Technology, “Biology of Symbiosis.”
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Tsui CKM, Vrijmoed LLP. A re-visit to the evolution and ecophysiology of the Labyrinthulomycetes. In: Cruzado A, editor. Marine ecosystems. InTech; 2012. p. 161–176.
- Raghukumar S. Thraustochytrid marine protists: production of PUFAs and other emerging technologies. Mar Biotechnol. 2008;10:631–640.10.1007/s10126-008-9135-4
- Fan KW, Chen F, Jones EB, et al. Eicosapentaenoic and docosahexaenoic acids production by and okara-utilizing potential of thraustochytrids. J Ind Microbiol Biotechnol. 2001;27:199–202.10.1038/sj.jim.7000169
- Nagano N, Taoka Y, Honda D, et al. Optimization of culture conditions for growth and docosahexaenoic acid production by a marine thraustochytrid, Aurantiochytrium limacinum mh0186. J Oleo Sci. 2009;58:623–628.10.5650/jos.58.623
- Kaya K, Nakazawa A, Matsuura H, et al. Thraustochytrid Aurantiochytrium sp. 18W-13a accumulates high amounts of squalene. Biosci Biotechnol Biochem. 2011;75:2246–2248.10.1271/bbb.110430
- Sijtsma L, de Swaaf ME. Biotechnological production and applications of the omega-3 polyunsaturated fatty acid docosahexaenoic acid. Appl Microbiol Biotechnol. 2004;64:146–153.10.1007/s00253-003-1525-y
- Jiang Y, Fan KW, Wong RT, et al. Fatty acid composition and squalene content of the marine microalga Schizochytrium mangrovei. J Agric Food Chem. 2004;52:1196–1200.10.1021/jf035004c
- Reddy LH, Couvreur P. Squalene: A natural triterpene for use in disease management and therapy. Adv Drug Delivery Rev. 2009;61:1412–1426.10.1016/j.addr.2009.09.005
- Rao CV, Newmark HL, Reddy BS. Chemopreventive effect of squalene on colon cancer. Carcinogenesis. 1998;19:287–290.10.1093/carcin/19.2.287
- Newmark HL. Squalene, olive oil, and cancer risk: a review and hypothesis. Cancer Epidemiol Biomarkers Prev. 1997;6:1101–1103.
- Oya S, Kanno D, Watanabe H, et al. Catalytic production of branched small alkanes from biohydrocarbons. ChemSusChem. 2015;8:2472–2475.10.1002/cssc.201500375
- Hernández-Pérez M, Gallego RMR, Alayón PJP, et al. Squalene content in livers from deep-sea sharks caught in Canary Island waters. Mar Freshwat Res. 1997;48:573–576.10.1071/MF97004
- He HP, Corke H, Cai JG. Supercritical carbon dioxide extraction of oil and squalene from amaranthus grain. J Agric Food Chem. 2003;51:7921–7925.10.1021/jf030488y
- Lack M, Sant G. Trends in global shark catch and recent developments in management. TRAFFIC Int. 2009;33.
- Salvador MD, Aranda F, Gomez-Alonso S, et al. Influence of extraction system, production year and area on Cornicabra virgin olive oil: a study of five crop seasons. Food Chem. 2003;80:359–366.10.1016/S0308-8146(02)00273-X
- Nakazawa A, Kokubun Y, Matsuura H, et al. TLC screening of thraustochytrid strains for squalene production. J Appl Phycol. 2014;26:29–41.10.1007/s10811-013-0080-x
- Nakayama T, Watanabe S, Mitsui K, et al. The phylogenetic relationship between the Chlamydomonadales and Chlorococcales inferred from 18SrDNA sequence data. Phycol Res. 1996;44:47–55.10.1111/pre.1996.44.issue-1
- Ueda M, Nomura Y, Doi K, et al. Seasonal dynamics of culturable thraustochytrids (Labyrinthulomycetes, Stramenopiles) in estuarine and coastal waters. Aquat Microb Ecol. 2015;74:187–204.10.3354/ame01736
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948.10.1093/bioinformatics/btm404
- Tamura K, Stecher G, Peterson D, et al. Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729.10.1093/molbev/mst197
