Abstract
Okaramines produced by Penicillium simplicissimum AK-40 activate l-glutamate-gated chloride channels (GluCls) and thus paralyze insects. However, the okaramine binding site on insect GluCls is poorly understood. Sequence alignment shows that the equivalent of residue Leucine319 of the okaramine B sensitive Bombyx mori (B. mori) GluCl is a phenylalanine in the okaramine B insensitive B. mori γ-aminobutyric acid-gated chloride channel of the same species. This residue is located in the third transmembrane (TM3) region, a location which in a nematode GluCl is close to the ivermectin binding site. The B. mori GluCl containing the L319F mutation retained its sensitivity to l-glutamate, but responses to ivermectin were reduced and those to okaramine B were completely blocked.
Fungi produce diverse secondary metabolites in response to environmental changes.Citation1,2) An intriguing example is the production by fungi of insect regulating metabolites when cultured on the soybean pulp “okara”. In 1989, Penicillium simplicissimum AK-40 cultured on okara was found to produce the indole alkaloids okaramines A and B (Fig. (A)), compounds showing insecticidal activity on larvae of the silkworm Bombyx mori.Citation3) Both okaramines possess a novel skeleton featuring the eight-membered azocine ring, and the pioneering studies inspired the total synthesis of okaramine N by Baran et al. at Harvard University.Citation4) Further exploration of insect-active fungal metabolites led to the isolation of insecticidal meroterpenoids, cyclic peptides as well as the other alkaloids.Citation5)
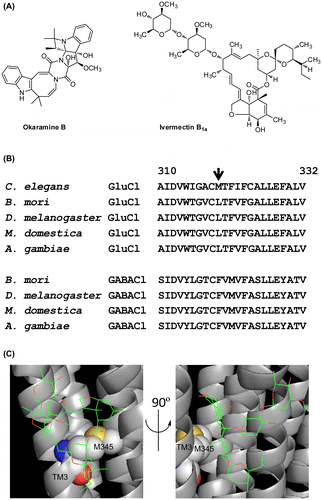
Fig. 1. Structures of Okaramine B and ivermectin B1a (A) and the alignment of amino acid sequences of C. elegans and insect GluCls and insect GABA-gated chloride channels (GABACls) (B).
Although successive discoveries of insect-modulating fungal metabolites were reported, their sites of action remained elusive, triggering investigations on possible targets. When tested on silkworm larvae, uncoordinated motility was rapidly observed, followed by paralysis or death, indicating that such compounds may modulate neural transmission.Citation5) Whole-cell patch-clamp electrophysiology was employed to show that whereas asperparaline ACitation6) and chrodrimaninsCitation7) blocked the acetylcholine- and γ-aminobutyric acid (GABA)-induced currents, respectively, okaramines induced chloride currents in neurons.Citation8) In particular, okaramines activated B. mori l-glutamate-gated chloride channels (BmGluCls), while having no effect on B. mori GABA-gated chloride channels (BmGABACls). The order of their GluCl activating potency (okaramine B > 4,5′-dihydrookaramine B > okaramine A > okaramine Q) correlated with their insecticidal potency, thereby supporting the view that the primary target of okaramines is GluCl.Citation8)
To explore further the site of action of okaramine B, we tested its capacity to displace the specific binding of [3H]ivermectin to membranes prepared from HEK293 cells expressing the exon 3c variant of BmGluCl. Okaramine B displaced [3H]ivermectin binding in a non-competitive fashion in that Bmax was reduced, whereas the Kd was unaffected, pointing to the possibility of a distinct site of binding from that of ivermectin.Citation9) However, this hypothesis requires further testing, because both classes of compounds activate GluCl, inducing persistent chloride currents in oocytes expressing BmGluCl.
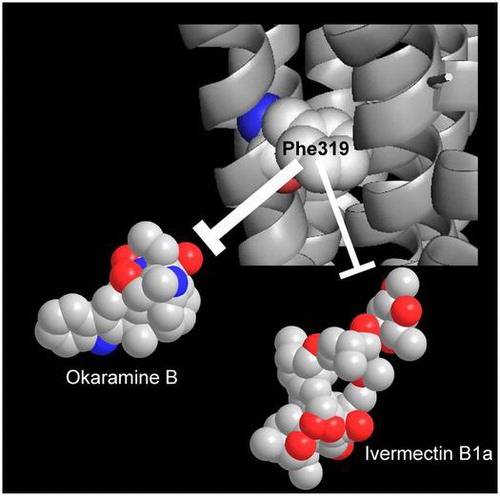
In the crystal structure of the Caenorhabditis elegans GluCl α-subunit in complex with ivermectin,Citation10) glycine342 and methionine345 in the third transmembrane region (TM3) (Fig. (B)) are located closest to ivermectin (Fig. (C)). Of these two amino acids, the glycine342 in the C. elegans GluCl is preserved in insect GluCls and GABACls, whereas the equivalent of methionine345 is leucine319 in the okaramine B-sensitive BmGluCl and phenylalanine319 in the okaramine B-insensitive B. mori GABA-gated chloride channel (BmGABACl) (Fig. (B)). Therefore, we investigated the effects of the L319F mutation in BmGluCl on the GluCl-activating action of okaramine B.
Materials and methods
Chemicals
Okaramine B was isolated from P. simplicissimum AK-40 metabolites generated when the fungus was grown on okara.Citation3) Ivermectin and sodium l-glutamate were purchased from Merck/Sigma–Aldrich (St. Louis, MO, USA) and Wako Pure Chemical Industries (Osaka, Japan), respectively.
cRNA preparation and injection into Xenopus laevis oocytes
The exon 3c variant of BmGluCl (accession number; KC342245)Citation11) was employed to investigate the actions of compounds in terms of inducing inward currents in oocytes expressing the BmGluCls, since the current amplitude of its response to l-glutamate and ivermectin is largest among all the splice variants.Citation11) The cDNA was cloned into the pcDNA3.1 (+) vector (Thermo Fisher Scientific, Waltham, MA, USA) and the nucleotide was mutated by polymerase chain reaction. Wild type and mutant cDNAs were linearized by cutting the 3′ end with BamHI and cRNA was prepared with the linearized cDNA using the mMESSAGE mMACHINE Ultra T7 kit (Thermo Fisher Scientific) as previously described.Citation11)
Oocytes were isolated from female Xenopus laevis anesthetized according to the U.K. Animals Act, 1986 and the follicle membranes were removed from oocytes after collagenase treatment.Citation12–14) Each oocyte was injected with 50 ng of cRNA and incubated in Standard Oocyte Saline (SOS) containing 100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.6) supplemented with antibiotics [penicillin (100 units ml−1), streptomycin (100 μg ml−1), gentamycin (20 μg ml−1)] and 2.5 mM sodium pyruvate for 24 h prior to recording GluCl response by two-electrode voltage-clamp electrophysiology.
Two-electrode voltage-clamp electrophysiology
Oocytes were perfused extracellularly at a flow rate of 7–10 ml min−1.Citation12–14) The transmembrane currents were recorded from oocytes using 3M KCl-filled glass microelectrodes (Resistance 0.5–5 MΩ) coupled to a GeneClamp 500B amplifier using Clampex 8 software (Molecular Devices, Sunnyvale, CA, USA). The currents were digitized using a Digidata1200 A/D converter (Molecular Devices) and analyzed using Clampfit 9 software (Molecular Devices). Oocytes were clamped at a holding potential (Eh) = −80 mV. The peak current amplitude of the response to each ligand was normalized to the peak current amplitude of the response to 100 μM l-glutamate. The concentration-normalized response data were fitted by non-linear regression with Prism 5 (GraphPad Software, La Jolla, CA, USA) according to Equation (1).(1)
In Equation (1), X is log[ligand concentration (M)], Imax is maximal normalized response, EC50 is half-maximal concentration and nH is the Hill coefficient. The Imax and pEC50 values for each ligand were obtained by repeated experiments (n = 4).
Results
l-Glutamate concentration-response curves for wild type and L319F mutant BmGluCl
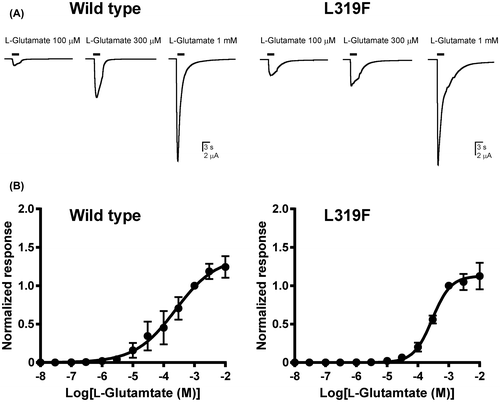
When bath-applied alone, l-glutamate resulted in concentration-dependent, inward currents at Eh –80 mV in oocytes expressing either wild type or L319F mutant BmGluCl (Fig. ). In the case of wild type BmGluCl, the concentration-response curve for l-glutamate reached a maximum at 100 μM with a pEC50 value of 3.61 ± 0.20 (Fig. (B), Table ). The L319F mutation did not significantly affect the pEC50 value of l-glutamate (3.54 ± 0.07) (Table ).
Table 1. pEC50 and Imax valuesCitationa for l-glutamate, ivermectin and okaramine B actions on wild type and L319F mutant BmGluCl expressed in Xenopus laevis oocytes.
Actions of ivermectin on wild type and L319F mutant BmGluCl
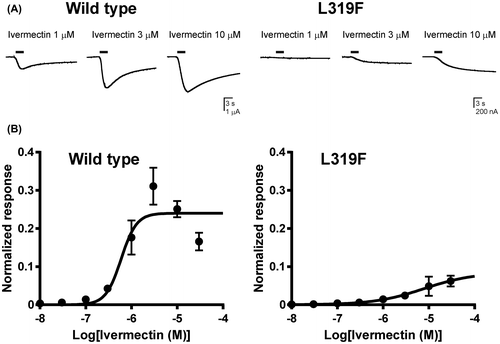
It is well documented that ivermectin primarily targets GluCls, although it has some modulatory actions on GABACls and related ligand-gated ion channels.Citation15) Ivermectin induced inward currents in Xenopus oocytes expressing the wild type and L319F mutant BmGluCl, with rise and decay times slower than those observed for l-glutamate-induced currents (Fig. (A)). At micromolar concentrations, such ivermectin actions were irreversible and thus a single oocyte was used to record only one ivermectin-induced response at each concentration. Using this protocol, the pEC50 and Imax values of ivermectin for the wild type GluCl were determined to be 6.23 ± 0.09 and 0.24 ± 0.02, respectively (Fig. (B)). The L319F mutation slowed desensitization of the ivermectin-induced response (Fig. (A)) and shifted both pEC50 and Imax values to 5.11 ± 0.71 and 0.08 ± 0.05, respectively (Fig. (B)) (Table ).
Fig. 2. Effects of the BmGluCl L319F mutation on the response to l-glutamate.
Actions of okaramine B on wild type and L319F mutant BmGluCl
Okaramine B was bath-applied to oocytes expressing the wild type and L319F mutant BmGluCl. It activated the wild type BmGluCl, inducing inward currents which were concentration-dependent (Fig. (A), (B)) with pEC50 and Imax values of 6.25 ± 0.10 and 0.19 ± 0.01, respectively (Table ). However, the compound was completely ineffective when tested on oocytes expressing the L319F mutant (Fig. (A), (B)) at concentrations from 10 nM to 100 μM. Thus, a pEC50 value could not be determined.
Fig. 3. Effects of the BmGluCl L319F mutation on the response to ivermectin.
Effect of co-application of okaramine B on the l-glutamate-induced response of the L319F mutant of BmGluCl
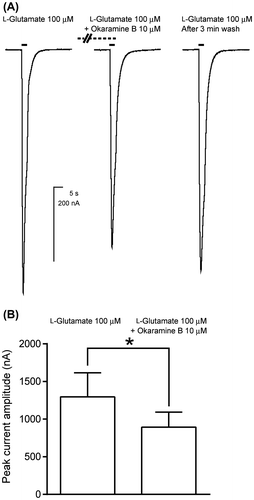
As shown in Fig. , okaramine B was incapable of activating the L319F mutant of BmGluCl. However, it could potentially act as antagonist or allosteric modulator on the mutant. Hence, okaramine B was applied at 10 μM for 1 min and then co-applied with 100 μM l-glutamate. This resulted in a 31% reduction of the peak current amplitude of the l-glutamate response (Figure , n = 4).
Fig. 4. Effects of the BmGluCl L319F mutation on the response to okaramine B.
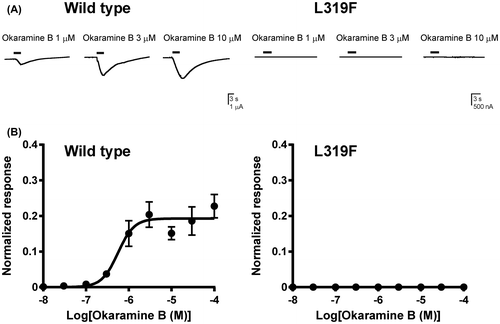
Fig. 5. Effects of co-application of okaramine B on the response to l-glutamate of BmGluCl expressed in Xenopus laevis oocytes.
Discussion
In this work, we have for the first time investigated the effects of the L319F mutation in TM3 of larval silkworm (B. mori) GluCl on the receptor’s sensitivity to l-glutamate, okaramine B and ivermectin. Based on structural studies on the C. elegans GluCl with ivermectin bound,Citation10) the residue equivalent to the one investigated here is likely also involved in the formation of the allosteric site. Thus, not surprisingly perhaps, the L319F mutation had no significant effect on the concentration-response curve of l-glutamate. The C. elegans studies of GluCl with l-glutamate bound showed binding of the natural ligand with the orthosteric site.Citation10) In contrast, the L319F mutation reduced the response to both ivermectin and okaramine B. The more profound effect was observed on the actions of okaramine B, the response to which was obliterated over the concentration range 10 nM–100 μM. The response to ivermectin was considerably reduced but concentration-dependent responses were still detected in the range 300 nM–30 μM. One plausible interpretation of this result is that okaramine B and ivermectin partly share a common allosteric binding site on BmGluCl but that bound okaramine B is located closer to leucine319 than is the case for bound ivermectin. Our previous observation that okaramine B displaced the binding of [3H] ivermectin to the membrane fractions containing BmGluCl in a non-competitive fashionCitation9) may be reconciled if the binding of okaramine B to one of five allosteric sites on BmGluCl attenuates the binding of ivermectin to the remaining four allosteric sites. To examine whether this is the case, it will be necessary to solve the X-ray crystal structure of an insect GluCl in complex with okaramine B.
The complete block of an activating response to okaramine B in the L319F mutant allowed us to address any possible modification (enhancement or block) by okaramine B of the response to l-glutamate. We detected a small but significant antagonist action in the L319F mutant. This suggests that whereas in the wild type BmGluCl occupation of the okaramine B binding site allows unimpaired coupling between the orthosteric binding site movement and channel opening, in the L319F mutant okaramine B can still bind but coupling between orthosteric site movement and channel opening in response to l-glutamate is attenuated. It should also be noted that the reduction by okaramine B of the peak response to l-glutamate in the L319F mutant of BmGluCl was only 31%, a not strong effect.
Since the L319F mutation hardly affects the response to l-glutamate, this may facilitate the generation of viable GluCl mutants of insects that are okaramine B- and ivermectin-resistant. Such mutants would be valuable in counter screens of small molecule mimics of okaramine B in the search for new insecticide leads. Our findings also suggest that it will be important to remain alert for the detection of resistance due to mutations in the equivalent residue to B. mori leucine319 in pest lepidoptera and other insect and acarine species exposed to abamectin and ivermectin. We have in the past pointed to the possibility of resistance emerging from mutations in loop D of the nicotinic acetylcholine receptor, which impact on neonicotinoids but not acetylcholineCitation13) and later such mutations were found in field populations.Citation16)
In conclusion, we have shown that a novel L319F mutation in TM3 of BmGluCl reduced okaramine B sensitivity more profoundly than its impact on ivermectin sensitivity, indicative of okaramine B interactions with GluCl in the vicinity of leicine319. In spite of these differences in their actions and the non-competitive actions of okaramine B in the displacement of [3H]ivermectin binding to the membrane fractions prepared from H293 cells expressing BmGluCl, further studies, in particular structural studies, will be needed to determine whether okaramine B interacts at a site distinct from that of ivermectin on BmGluCl.
Author contributions
Kazuhiko Matsuda designed the study and wrote the manuscript; Shogo Furutani performed the experiments and wrote the manuscript; Daiki Okuhara and Anju Hashimoto performed the experiments; Makoto Ihara performed the data analysis and wrote the manuscript; Kenji Kai and Hideo Hayashi provided okaramine B and wrote manuscript; David B. Sattelle discussed the results and wrote the manuscript. All the authors approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was supported by the Grant-in-Aid for Scientific Research (A) [grant number 17H01472] from the Japan Society for the Promotion of Science.
Notes
Abbreviations: Bombyx mori l-Glutamate-gated chloride channel, BmGluCl; Bombyx mori γ-aminobutyric acid-gated chloride channel, BmGABACl; third transmembrane (TM3).
References
- Bode HB, Bethe B, Höfs R, et al. Big effects from small changes: possible ways to explore nature's chemical diversity. ChemBioChem. 2002;3(7):619–627.10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9
- Keller NP, Turner G, Bennett JW. Fungal secondary metabolism – from biochemistry to genomics. Nat Rev Microbiol. 2005;3(12):937–947.10.1038/nrmicro1286
- Hayashi H, Takiuchi K, Murao S, et al. Structure and insecticidal activity of new indole alkaloids, okaramines A and B, from Penicillium simplicissimum AK-40. Agric Biol Chem. 1989;53(2):461–469.
- Baran PS, Guerrero CA, Corey EJ. Short, enantioselective total synthesis of okaramine N. J Am Chem Soc. 2003;125(19):5628–5629.10.1021/ja034491+
- Hayashi H. Frontier studies on highly selective bio-regulators useful for environmentally benign agricultural production. Biosci Biotechnol Biochem. 2015;79(6):877–887.10.1080/09168451.2015.1015954
- Hirata K, Kataoka S, Furutani S, et al. A fungal metabolite asperparaline a strongly and selectively blocks insect nicotinic acetylcholine receptors: the first report on the mode of action. PLoS ONE. 2011;6(4):e18354.10.1371/journal.pone.0018354
- Xu Y, Furutani S, Ihara M, et al. Meroterpenoid chrodrimanins are selective and potent blockers of insect GABA-gated chloride channels. PLOS ONE. 2015;10(4):e0122629.10.1371/journal.pone.0122629
- Furutani S, Nakatani Y, Miura Y, et al. GluCl a target of indole alkaloid okaramines: a 25 year enigma solved. Sci Rep. 2014;4:6190.
- Furutani S, Ihara M, Kai K, et al. Okaramine insecticidal alkaloids show similar activity on both exon 3c and exon 3b variants of glutamate-gated chloride channels of the larval silkworm, Bombyx mori. Neurotoxicology. 2017;60:240–244.
- Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474(7349):54–60.10.1038/nature10139
- Furutani S, Ihara M, Nishino Y, et al. Exon 3 splicing and mutagenesis identify residues influencing cell surface density of heterologously expressed silkworm (Bombyx mori) glutamate-gated chloride channels. Mol Pharmacol. 2014;86(6):686–695.10.1124/mol.114.095869
- Shimomura M, Okuda H, Matsuda K, et al. Effects of mutations of a glutamine residue in loop D of the α7 nicotinic acetylcholine receptor on agonist profiles for neonicotinoid insecticides and related ligands. Br J Pharmacol. 2002;137(2):162–169.10.1038/sj.bjp.0704848
- Shimomura M, Yokota M, Ihara M, et al. Role in the selectivity of neonicotinoids of insect-specific basic residues in loop D of the nicotinic acetylcholine receptor agonist binding site. Mol Pharmacol. 2006;70(4):1255–1263.10.1124/mol.106.026815
- Ihara M, Matsuda K, Otake M, et al. Diverse actions of neonicotinoids on chicken α7, α4β2 and Drosophila-chicken SADβ2 and ALSβ2 hybrid nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Neuropharmacology. 2003;45(1):133–144.10.1016/S0028-3908(03)00134-5
- Wolstenholme AJ. Recent progress in understanding the interaction between avermectins and ligand-gated ion channels: putting the pests to sleep. Ivert Neurosci. 2010;10(1):5–10.10.1007/s10158-010-0105-y
- Bass C, Puinean AM, Andrews M, et al. Mutation of a nicotinic acetylcholine receptor β subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. BMC Neurosci. 2011;12:51.10.1186/1471-2202-12-51
