Abstract
This study investigated the effects of high-intensity ultrasound and glycosylation on the structural and interfacial properties of the Maillard reaction conjugates of buckwheat protein isolate (BPI). The covalent attachment of dextran to BPI was confirmed by examination of the Fourier-transform infrared spectra. Emulsifying properties of the conjugates obtained by ultrasound treatment were improved as compared to those obtained by classical heating. Structural feature analyses suggested that conjugates obtained by ultrasound treatment had less α-helix and more random coil, higher surface hydrophobicity and less compact tertiary structure as compared to those obtained by classical heating. The surface activity measurement revealed that the BPI–dextran conjugates obtained by ultrasound treatment were closely packed and that each molecule occupied a small area of the interface. Combination of ultrasonic treatment and glycosylation was proved to be an efficient way to develop new stabilizers and thickening agents for food in this study.
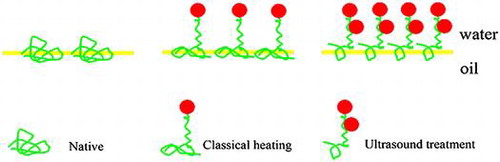
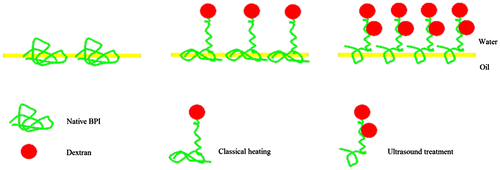
Supposed mechanism of adsorbed layers at oil/water interface for protein–polysaccharide conjugates obtained by classical heating or ultrasound treatments.
Buckwheat (Fagopyrum esculentum Moench) has great potential as a functional ingredient in the food industry and is also known as a valuable source of proteinCitation1). The proteins in buckwheat have recently attracted much interest, due to their well-balanced amino acid composition and potential health effects, such as hypolipidemic activityCitation2), cholesterol-lowering, and enhanced fecal steroid excretion effectsCitation1,3), chemopreventive activity against mammary tumorigenesis and colon carcinogenesisCitation4,5). The content of protein in buckwheat flour is ranging from 8.51 to 18.87% depending on variety. The albumin and globulin fractions constitute the major part of buckwheat protein isolate (BPI)Citation6).
To utilize the dietary protein as ingredients in food industry, it is necessary to determine the physicochemical and functional properties of the proteins. There are several reports concerning the functional properties of buckwheat protein productsCitation7,8). According to those reports, the buckwheat protein has some superior functional properties, such as higher nitrogen solubility index and higher water holding, emulsifying and foaming capacities comparing with soy protein isolate. However, the improvement of functional properties of buckwheat protein has not been fully investigated.
The functional properties of proteins are of key interest to manufacturers of pharmaceutical, food, and cosmetic products. However, native vegetable proteins in general are of limited functionality, including emulsifying and other surface properties. Many modifications have been used to improve the functional properties of proteins, such as chemical, physical, enzymatic treatments, or the combination thereof. Results from several studies showed that the functional properties of proteins were improved through protein–polysaccharide graft reactions, which are based on Maillard reactionsCitation9,Citation10). The most striking characteristic of the resulting protein–polysaccharide conjugates is the excellent emulsifying propertiesCitation10). In addition to the improvement of emulsifying properties, this method of modification was efficient in improving the solubility, antibacterial effect, and antioxidant effect of proteinsCitation11–13). It has also been proposed that conjugation of the allergen protein with polysaccharides may be effective in reducing the allergenicityCitation14). Moreover, this method utilized a naturally occurring reaction and no chemical reagent was applied. Therefore, this method could be one of the most promising approaches for food applications because of its safety.
The graft reactions are based on Maillard reactions between the ε-amino groups of proteins and the reducing-end carbonyl groups of polysaccharides. The efficiency of reactions is affected by tertiary conformation, spatial structure, and surface properties of protein molecules. The approach which could cause more groups and regions inside the molecules to expose or induce molecular unfolding of protein might be helpful for improving the efficiency of graft reactions. The ultrasound has been widely used in food industry due to its promising effects in food and product modificationCitation15). Ultrasound can accelerate chemical reactions, increase diffusion rates, and disperse aggregates through acoustic cavitationCitation16). Certain studies investigated the changes in molecular structure after high-intensity ultrasound treatment, which induced alterations in free sulfhydryl groups, particle sizes, surface hydrophobicity, and secondary structures of proteinsCitation17) and an increase in intramolecular mobility and surface activityCitation18). Similar study also reported that ultrasonic treatment resulted in partial unfolding and reduction of intermolecular interactions of proteins, as demonstrated by increase in free sulfhydryl groups and surface hydrophobicityCitation19,20). Therefore, after the ultrasound procedure, the residues of unfolded proteins were exposed outward and might became highly reactive to the reducing-end carbonyl groups of polysaccharides with a little steric hindrance compared with folded proteins. Our previous studies have shown that ultrasound could accelerate the graft reaction and conjugates prepared by ultrasound treatment showed better emulsifying properties than those obtained by classical heatingCitation21). However, limited information is available so far about its mechanism.
The overall objective of this study, therefore, was to investigate the effects of ultrasound treatment on the structural and interfacial properties of the Maillard reaction conjugates of BPI. The modified BPI was analyzed for net charge, hydrophobicity, surface properties, and emulsifying stability.
Materials and methods
Materials and chemicals
Dextran (MW 40,000) was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China), which was used without further purification. 1, 8-anilinonaphthalenesulfonate (ANS) regent was purchased from Sigma (St. Louis, MO, USA). Bovine serum albumin (BSA) and all other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Soybean oil was purchased from local supermarket and used directly.
Preparation of BPI
BPI was prepared from buckwheat flour purchased from a local supermarket. The flour (500 g) was treated with 5 L n-hexane/ethanol (10:1, v/v) to extract oil. The defatted meal (250 g) was dispersed in distilled water (5 L) and adjusted to pH 8.0. The dispersion was stirred for 2 h to extract proteins and then centrifuged at 4000 g for 30 min at 4 °C. The supernatant was adjusted to pH 4.5 and centrifuged at 4000 g for 20 min at 4 °C. The pellet was washed twice with distilled water. Thereafter, the protein pellet was resuspended in distilled water and neutralized to pH 7.0. The samples were freeze-dried and stored in a 4 °C cooler.
Preparation of the BPI–dextran conjugates
BPI (1%, w/v) and dextran (1%, w/v) were dispersed in phosphate buffer solution (0.2 M, pH7.5). Then the 100 mL of solution was treated by an ultrasonic equipment (VC × 500, Sonics vibra cell, USA) with a probe of a 10 mm titanium tip for 80 min at 70 °C and ultrasonic intensity of 544.59 W/m2. Thereafter, the slurry was cooled to ambient temperature and dialyzed at 4 °C for 24 h. Finally, the samples were freeze-dried and stored at room temperature. The same solution was conducted by classical heating in a JR-AB water bath oscillator (Jerel electric appliance Co., Ltd., Jiangsu, China) at 180 rpm and 70 °C for 40 h. The subsequent procedure was the same to ultrasonic treatment.
Fourier transform infrared spectroscopy
The Fourier transform infrared (FT-IR) spectra of BPI and BPI conjugated with dextran were recorded with a Nicolet NEXUS spectrometer (Nicolet Co., USA). The freeze-dried samples (1.0 mg) were mixed with spectroscopic grade potassium bromide (KBr) powder at a ratio of 1:30, ground, then pressed into a 1 mm pellet by employing a stainless-steel cup (diameter 10 mm) prior to FT-IR measurement. A total of 64 scans were performed at 4 cm−1 resolution. Scanning was carried out in the range 4000−400 cm−1.
Analysis of circular dichroism spectrum
Conformational changes in the secondary structure of protein samples were analyzed using a Mos-450 CD spectropolarimeter (Biologic, Claix, France) at 25 °C. Secondary structure determination was performed by scanning diluted protein samples (0.2 mg/mL in protein, diluted with 10 mM phosphate buffer solution, pH 7.0) between 190 and 250 nm. The same phosphate buffer solution was used as blank solution for all samples. Five scans were averaged to obtain one spectrum.
Intrinsic fluorescence emission spectroscopy
Intrinsic emission fluorescence spectra of the protein samples were obtained by a Hitachi F-7000 fluorescence spectrophotometer (Hitachi, Ltd., Tokyo, Japan). Protein solutions (0.15 mg/mL) were prepared in 10 mM phosphate buffer (pH 7.0). The protein solutions were excited at 290 nm (slit width 5 nm) and emission spectra were recorded from 300 to 400 nm at a 10 nm/s scanning speed.
Surface hydrophobicity (H0)
Surface hydrophobicity was measured using the ANS as the fluorescence probeCitation22). Protein dispersions were diluted (0.05, 0.1, 0.2, 0.5, 1, and 2 mg/mL) in phosphate buffer solution (0.2 M, pH 7.5). Then, an aliquot of ANS solution (20 μL, 8.0 mM in the same buffer) was added to 4 mL of sample. Fluorescence intensity (FI) was measured with a Hitachi F-7000 fluorescence spectrometer (Hitachi, Ltd., Tokyo, Japan) at wavelengths of 390 nm (excitation) and 470 nm (emission). The initial slope of the FI vs. protein concentration plot (calculated by linear regression analysis) was used as an index of protein hydrophobicity.
Solubility
Solubility was determined by dispersing the samples in distilled water to obtain a final solution of 2 mg/mL in protein. The protein solution was centrifugated at 12,000g for 30 min (4 °C). The content of protein for the resulting solution was analyzed according to the Lowry methodCitation23).
Emulsion activity and stability
The emulsifying activity index (EAI) and emulsifying stability index (ESI) were measured according to the method of Wang et al.Citation24). For emulsion formation, 5 mL of soy oil and 15 mL of sample solution (2 mg/mL in protein) in de-ionized water (pH 7.0) were homogenized in a PT-MR 2100 (KINEMATICA AG, Switzerland) for 1 min at 24000 rpm. Fifty microliter of emulsion were taken from the bottom of the homogenized emulsion, immediately (0 min) or 10 min after homogenization, and diluted (1:100, v/v) in 0.1% (w/v) SDS solution. After shaking in a vortex mixer for 5 s, the absorbance of diluted emulsions was recorded at 500 nm using UV–vis spectrophotometer (Shanghai Spectrum Instruments Co., Ltd., China). EAI was calculated using the equation:
where A0 represents the absorbance of the diluted emulsions at 0 min, C is the protein concentration (g/mL) before emulsification, φ is the oil volume fraction (v/v) of the emulsion and θ is the optical path (1 cm).
ESI was defined as:
where A0 and A10 represent the absorbance of the diluted emulsions at 0 and 10 min, respectively.
Emulsion droplet size distribution
The continuous phase (2% for samples, w/w) was prepared by dissolving the sample into deionized water. The pH of the solutions was adjusted to 7.5. Oil-in-water emulsion was prepared by blending 10% soybean oil (v/v) and 90% sample solution (v/v) together using a pressure homogenizer (AH2010, ATS Engineering, Inc., Shanghai, China) at 30 MPa. Two passes were used to ensure uniform mixing of the oil and protein solutions. The oil droplet size distributions were analyzed with dynamic light scattering using a Malvern Mastersizer 2000 unit (Malvern Instruments Ltd., Malvern, U.K.). The droplet size distribution of emulsions stored at room temperature was measured for different times (0 and 7 days).
Zeta-potential determination
The zeta-potential of the samples was determined with a nanoZS instrument (Malvern Instruments, Worcestershire, UK). The samples were diluted to a concentration of about 0.001 wt% to avoid multiple scattering effects.
Surface tension measurement
The surface tension (γ) of the interface stabilized by the BPI–polysaccharide was determined by a DCAT21 automated surface tension meter (Dataphysics, Berlin, Germany). All experiments were performed at 20 °C. The critical micelle concentration (CMC) was then automatically calculated. The surface excess concentration (Γ), the area occupied by each molecule (A), was calculated from the CMC and γ values. According to the Gibbs adsorption Equation:
where C is the concentration, R is the gas constant, and T is the absolute temperature. The mean area occupied per molecule of the adsorbed surfactant, A, was calculated according to
where N is Avogadro’s constant.
Statistical analysis
All the experiments were performed in triplicate and the data obtained were analyzed by one-way analysis of variance (One-Way ANOVA) using SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL). Values are expressed as means ± SD. Duncan’s multiple range test was used to identify significant differences (p < 0.05) between means.
Results and discussion
FTIR spectroscopy
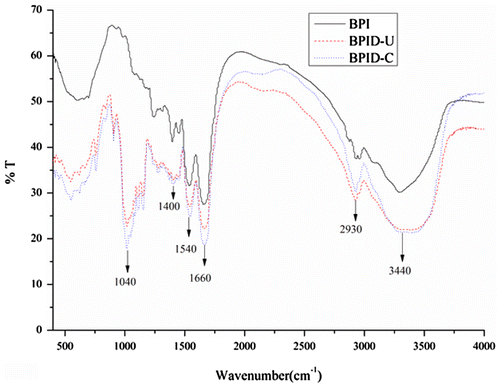
The FTIR spectrums of solid BPI and BPI–dextran conjugates are shown in Fig. . The FTIR spectrums exhibited characteristic bands at 1040 and 1400 cm−1, mainly related to C–O and C–N stretching vibrations, respectively. The strong adsorption in this region indicated that dextran was attached to the BPI. Bands at 2930 and 3440 cm−1 correlated with –C–H(sp3) antisymmetric stretching vibration and –O–H stretching vibration. The absorbance in these areas in the BPI–dextran conjugates was greater than that in the BPI. The bands at 1660 and 1540 cm−1 referred to C=O association stretching and –N–H plane bending vibration, known as the amide 1 and 2 bands, respectivelyCitation25). The adsorption of the amide 2 band increased in the BPI–dextran conjugates. The results also indicated that glycoconjugates were formed by covalent bonds.
Effects of combined modification on structures
The secondary structure of BPI and BPI–dextran conjugates (grafted BPI) was determined using CD spectroscopy. As shown in Table , compared with the BPI, grafted BPI showed the decrease in the content of α-helical structure. The decrease of α-helix is considered to be polysaccharides bound to ε-amino group in the α-helix regionCitation26). This result indicated that the attachment of polysaccharides to BPI led to changes in spatial structure and unfolding of protein molecules. Furthermore, conjugates obtained by ultrasonic treatments lost more α-helix, which indicated that ultrasound-assisted grafted BPI showed more evident change in the distribution of secondary structure. These changes in secondary structure made conjugates obtained by ultrasonic treatment show more uniformity and flexibility, which are helpful for the explanation of functionality changes of BPI.
Table 1. Secondary structure distribution of BPI and conjugates.
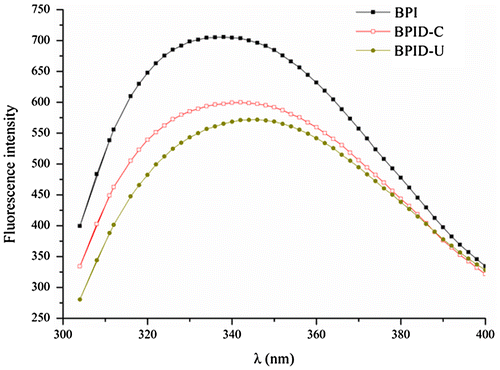
The changes in the tertiary structure of the protein can be determined from the fluctuations of the fluorescenceCitation27). It can be seen from Fig. that, the grafted BPI showed a lower λmax values and substantial significant shift of λmax to longer wavelengths (bathochromic shift) relative to that of BPI. The bathochromic shift of the maximal-emission wavelengths indicated that the Trp side chains moved to the outside of the protein molecules, which causes the polarity of their microenvironment to increaseCitation28). This result indicated that conformation of BPI has been changed after glycosylation and the conjugates had much less compact tertiary conformation than BPICitation22). Furthermore, the shape of fluorescence spectrum and λmax of conjugates obtained by ultrasonic treatments were different from conjugates obtained by classical heating. This result also indicated that the tertiary structure of conjugates obtained by ultrasonic treatments is different from those obtained by classical heating.
Effects of combined modification on H0, solubility, and emulsifying properties
It is well known that surface hydrophobicity influences protein on functionality to a great extentCitation29). The H0 values of samples are shown in Fig. . The graft reaction between BPI and dextran could significantly (p < 0.05) increase the H0 value of BPI. This result indicated that the conjugates had markedly higher surface hydrophobicity than BPI, probably due to aggregate dissociation or protein unfolding. This result also confirmed that steric structure of BPI changed while polysaccharide molecules were linked to the proteinsCitation30). Furthermore, H0 values of conjugates obtained by ultrasonic treatment were higher than that of conjugates obtained by classical heating. This result indicated that ultrasound-assisted grafted BPI possessed more hydrophobic residues toward the aqueous environment.
Fig. 3. Surface hydrophobicity (A), solubility (B) and emulsifying properties (C and D) of BPI and BPI–dextran conjugates.
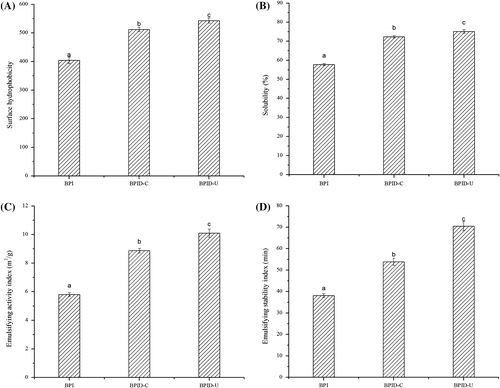
Solubility is the practical measure of protein denaturation and aggregation, and hence it can influence other functional properties, such as emulsifying property. The solubility of BPI was significantly improved after grafting with dextran (as shown in Fig. ) in spite of the increase in the surface hydrophobicity. Normally, when hydrophobic groups were exposed, the protein molecules might reform macromolecular aggregates through noncovalent interactions, which results in a reduction in solubility. In fact, protein gained more hydrophilic groups after grafting with dextranCitation31). On the other hand, the attachment of dextran might also inhibit the noncovalent interactions between proteins, which lead to increased protein solubility. Furthermore, BPI–dextran conjugates obtained by ultrasonic treatment showed better solubility than those obtained by classical heating. This result indicated the previous one had more hydrophilic groups and ultrasonic treatment increased protein–water interactions.
Emulsifying property of protein is essentially determined by their physicochemical and conformational propertiesCitation32) and is related to the protein solubility, surface charge, surface hydrophobicity, and molecular flexibility. EAI and ESI of BPI and modified samples are shown in Fig. . As shown in Fig. , the graft reaction between BPI and dextran could significantly (p < 0.05) increase the EAI and ESI of BPI. It has been proved that formation of protein–polysaccharide conjugates combines the characteristic property of proteins to adsorb strongly to the oil–water interface with the characteristic property of the polysaccharides for solvation in the aqueous phase mediumCitation33). The EAI and ESI of conjugates obtained by ultrasonic treatment were improved as compared with those obtained by classical heating. This phenomenon might be attributed to the improvement of solubility of conjugates (as shown in Fig. ). It has been reported that solubility is a determining factor in the ability to form emulsionsCitation34). On the other hand, high-intensity ultrasonic treatment was capable of increasing molecules mobility and makes the sample absorb in the oil/water interface much faster due to mechanical effects associated with cavitation by temporarily dispersing aggregates and breaking covalent bonds in polymeric chainsCitation35).
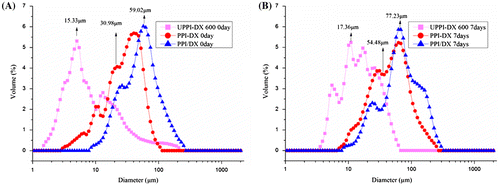
The comparative droplet size distributions of BPI and BPI–dextran conjugates obtained by different treatments are shown in Fig. . Emulsions stabilized by BPI–dextran conjugates gained by ultrasonic treatment presented a lower particle size than those of BPI–dextran conjugates obtained by classical heating. Moreover, BPI–dextran conjugates obtained by ultrasonic treatment gave emulsions more stability against creaming than those prepared with BPI–dextran conjugates obtained by classical heating during storage at ambient temperature (seven days).
Fig. 4. The droplet size distribution of BPI and BPI–dextran conjugates during ambient temperature storage (A: 0 day or B: 7 days).
Effects of combined modification on zeta-potential
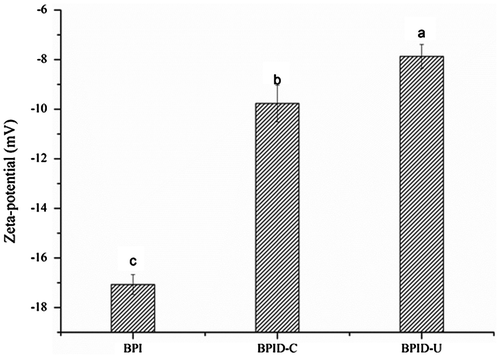
The surface charge of adsorbed surfactants expressed as the zeta-potential is known to play an important role in stabilization of an emulsion. Fig. shows the zeta-potential values of BPI and conjugates dispersions. Obviously, the zeta-potential values of the conjugates were all higher than that of BPI. A significant decrease in the negative zeta-potential values of the conjugates obtained by ultrasonic treatment compared with that of the conjugates obtained by classical heating. The results suggested that the addition of polysaccharide changed the distribution of the BPI’s surface charge. Electrostatic screening effects might be responsible for the decrease in the zeta-potential valuesCitation29).
Effects of combined modification on surface properties
As shown in Table , glycation resulted in an increase in the surface excess and a decrease in the size of the interface. In addition, the conjugates obtained by ultrasonic treatment had a maximum Γ value and a minimum A value, indicating that the conjugates obtained by ultrasonic treatment were closely packed and that each molecule occupied a smaller area of the interface than in those obtained by classical heating.
Table 2. Surfactant properties of BPI–polysaccharide conjugates, Γ and A, were calculated from the critical micelle concentration (CMC) and γ.
Supposed mechanism for the effect of ultrasound treatment on the conjugates adsorbed layers at oil/water interface
It is well known that protein–polysaccharide graft reactions are based on Maillard reactions between the free amino groups of proteins and the reducing-end carbonyl groups of polysaccharides. The ultrasonic treatment was helpful for giving more free amino groups for graft reaction, as proved by previously studyCitation36). The thermal, mechanical, and chemical effects of ultrasound treatment are attributed to the rapid formation and collapse of cavitational bubbles in liquid at high water activity. These bubbles collapse in the positive pressure cycle and produce highly turbulent flowing conditions and extremely high pressure and temperatureCitation37). This leads to unfolding of protein and supporting more free amino groups for the process of graft reaction. The unfolding of protein induced by ultrasound treatment can be proved by decreased fluorescence intensityCitation38), change in secondary structureCitation39), increase in the number of hydrophobic groupsCitation38), and increase in protein solubilityCitation40,41). Ultrasound irradiation could cause more groups and regions (such as hydrophilic amino acid residues) inside the molecules to expose, disrupt the interactions between local sequence stretches of amino acids and between distinct parts of the molecule, destroy hydrophobic interactions of protein molecules and break peptide bonds. All those factors would be helpful for understanding the fact that ultrasound treatment could accelerate the graft reaction.
It has been reported that proteins alone tended to form a monolayer at the interface for biopolymer complexes, and that different structures of mixed layers formed depending on the neutral or negative charge of the complexCitation42). Actually, the final interfacial properties of the Maillard reaction products were determined by the combined effects of the amount of polysaccharide attached to the protein, electrostatic repulsion, hydrophobic interaction, and the coverage of protein in the interface. We harvested informative data providing a comprehensive insight to the interfacial properties of BPI, a schematic diagram was proposed in Fig. . The figure shows that BPI alone formed loose adsorption at the interface and each molecule occupied a large area. The glycation with polysaccharide could reduce the adsorption sites and that each molecule occupied a smaller area than with BPI, whereas the amount of adsorption increased. The conjugates obtained by ultrasonic treatment occupied a small area at the interface and tended to be closely packed and form a thick interfacial film, probably because significant electrostatic shielding decreased the repulsion between BPICitation43).
Conclusion
In summary, this study elucidated the effects of ultrasonic treatment and glycosylation on the structure and interfacial properties of BPIs. The final functional properties of the Maillard reaction products were determined by combined effects of ultrasonic treatment and glycosylation. When used as surfactants, conjugates obtained by ultrasonic treatment showed the better emulsifying property, probably because they occupied a small area at the interface and tended to be closely packed and form a thick interfacial film. Further study is required to investigate the physicochemical properties of the adsorbed layer and its stability regarding changes in temperature, pH value, and enzymes.
Author contributions
Feng Xue conceived and designed the experiments; Zhoushan Wu and Jinrong Tong performed the experiments; Jialun Zheng analyzed the data; Chen Li wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was financially supported by the National Natural Science Foundation of China [grant number NSFC31501529, NSFC31401650 and NSFC30972044]; Jiangsu Provincial Natural Science Foundation [grant number BK20150950, BK20171066] and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Li SQ, Zhang QH. Advances in the development of functional foods from buckwheat. Crit Rev Food Sci Nutr. 2001;41:451–464.10.1080/20014091091887
- Tomotake H, Kayashita J, Kato N. Hypolipidemic activity of common (Fagopyrum esculentum Moench) and tartary (Fagopyrum tataricum Gaertn.) buckwheat. J Sci Food Agric. 2015;95:1963–1967.10.1002/jsfa.2015.95.issue-10
- Krkoskova B, Mrazova Z. Prophylactic components of buckwheat. Food Res Int. 2005;38:561–568.10.1016/j.foodres.2004.11.009
- Liu Z, Ishikawa W, Huang X, et al. A buckwheat protein product suppresses 1,2-dimethylhydrazine-induced colon carcinogenesis in rats by reducing cell proliferation. J Nutr. 2001;131:1850–1853.
- Kayashita J, Shimaoka I, Nakajoh M, et al. Consumption of a buckwheat protein extract retards 7,12-dimethylbenz[α] anthracene-induced mammary carcinogenesis in rats. Biosci Biotechnol Biochem. 1999;63:1837–1839.10.1271/bbb.63.1837
- Tang C-H, Peng J, Zhen D-W, et al. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem. 2009;115:672–678.10.1016/j.foodchem.2008.12.068
- Bejosano FP, Corke H. Properties of protein concentrates and hydrolysates from Amaranthus and Buckwheat. Ind Crops Prod. 1999;10:175–183.10.1016/S0926-6690(99)00021-7
- Tomotake H, Shimaoka I, Kayashita J, et al. Physicochemical and functional properties of buckwheat protein product. J Agric Food Chem. 2002;50:2125–2129.10.1021/jf011248q
- Kato A. Industrial applications of maillard-type protein-polysaccharide conjugates. Food Sci Technol Res. 2002;8:193–199.10.3136/fstr.8.193
- Oliveira, FCd, Coimbra, JSdR, de Oliveira, EB, et al. Food protein-polysaccharide conjugates obtained via the maillard reaction: A review. Crit Rev Food Sci Nutr. 2014; 56(7), 1108–1125.
- Xue F, Li C, Zhu X, et al. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res Int. 2013;51:490–495.10.1016/j.foodres.2013.01.012
- Amiri S, Ramezani R, Aminlari M. Antibacterial activity of dextran-conjugated lysozyme against Escherichia coli and Staphylococcus aureus in cheese curd. J Food Protect. 2008;71:411–415.10.4315/0362-028X-71.2.411
- Fan JF, Zhang YY, Szesze T, et al. Improving functional properties of soy protein hydrolysate by conjugation with curdlan. J Food Sci. 2006;71:C285–C291.
- Arita K, Babiker EE, Azakami H, et al. Effect of chemical and genetic attachment of polysaccharides to proteins on the production of IgG and IgE. J Agric Food Chem. 2001;49:2030–2036.10.1021/jf001120t
- Arzeni C, Pérez OE, Pilosof AMR. Functionality of egg white proteins as affected by high intensity ultrasound. Food Hydrocoll. 2012;29:308–316.10.1016/j.foodhyd.2012.03.009
- Gogate PR. Cavitational reactors for process intensification of chemical processing applications: a critical review. Chem Eng Process. 2008;47:515–527.10.1016/j.cep.2007.09.014
- Gülseren İ, Güzey D, Bruce BD, et al. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason Sonochem. 2007;14:173–183.10.1016/j.ultsonch.2005.07.006
- Guzey D, Gulseren I, Bruce B, et al. Interfacial properties and structural conformation of thermosonicated bovine serum albumin. Food Hydrocoll. 2006;20:669–677.10.1016/j.foodhyd.2005.06.008
- Hu H, Fan X, Zhou Z, et al. Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrason Sonochem. 2013;20:187–195.10.1016/j.ultsonch.2012.07.011
- Zhang QT, Tu ZC, Xiao H, et al. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Bioprod Process. 2014;92:30–37.10.1016/j.fbp.2013.07.006
- Li C, Xue H, Chen Z, et al. Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res Int. 2014;57:1–7.
- Liu Y, Zhao G, Zhao M, et al. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2012;131:901–906.10.1016/j.foodchem.2011.09.074
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Wang J, Zhao M, Jiang Y. Effects of wheat gluten hydrolysate and us ultrafiltration fractions on dough properties and bread quality. Food Technol Biotechnol. 2007;45:410–414.
- Zhang Q-T, Tu Z-C, Xiao H, et al. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food and Bioprod Process. 2014;92:30–37.10.1016/j.fbp.2013.07.006
- Hattori M, Nagasawa K, Ametani A, et al. Functional changes in.beta.-lactoglobulin by conjugation with carboxymethyl dextran. J Agric Food Chem. 1994;42:2120–2125.10.1021/jf00046a009
- He W, Tan Y, Tian Z, et al. Food protein-stabilized nanoemulsions as potential delivery systems for poorly water-soluble drugs: preparation, in vitro characterization, and pharmacokinetics in rats. Int J Nanomedicine. 2011;6:521–533.
- Pallarès I, Vendrell J, Avilés FX, et al. Amyloid fibril formation by a partially structured intermediate state of alpha-chymotrypsin. J Mol Biol. 2004;342:321–331.10.1016/j.jmb.2004.06.089
- Chandrapala J, Zisu B, Palmer M, et al. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason Sonochem. 2011;18:951–957.10.1016/j.ultsonch.2010.12.016
- Guan JJ, Qiu AY, Liu XY, et al. Microwave improvement of soy protein isolate-saccharide graft reactions. Food Chem. 2006;97:577–585.10.1016/j.foodchem.2005.05.035
- Jiménez-Castaño L, Lopez-Fandino R, Olano A, et al. Study on β-lactoglobulin glycosylation with dextran: effect on solubility and heat stability. Food Chem. 2005;93:689–695.10.1016/j.foodchem.2004.09.050
- Tang C-H, Sun X, Foegeding EA. Modulation of physicochemical and conformational properties of kidney bean vicilin (Phaseolin) by glycation with glucose: implications for structure-function relationships of legume vicilins. J Agric Food Chem. 2011;59:10114–10123.10.1021/jf202517f
- Dickinson E, Galazka VB. Emulsion stabilization by ionic and covalent complexes of β-lactoglobulin with polysaccharides. Food Hydrocoll. 1991;5:281–296.10.1016/S0268-005X(09)80114-8
- Akhtar M, Dickinson E. Emulsifying properties of whey protein–dextran conjugates at low pH and different salt concentrations. Colloids Surf B Biointerfaces. 2003;31:125–132.10.1016/S0927-7765(03)00049-3
- Kjartansson GT, Zivanovic S, Kristbergsson K, et al. Sonication-assisted extraction of chitin from North Atlantic shrimps (Pandalus borealis). J Agric Food Chem. 2006;54:5894–5902.10.1021/jf060646w
- Mu L, Zhao M, Yang B, et al. Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. J Agric Food Chem. 2010;58:4494–4499.10.1021/jf904109d
- Shirsath SR, Sonawane SH, Gogate PR. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem Eng Process. 2012;53:10–23.10.1016/j.cep.2012.01.003
- Subhedar PB, Gogate PR. Enhancing the activity of cellulase enzyme using ultrasonic irradiations. J Mol Catal B-Enzym. 2014;101:108–114.10.1016/j.molcatb.2014.01.002
- Jiang L, Wang J, Li Y, et al. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res Int. 2014;62:595–601.10.1016/j.foodres.2014.04.022
- Chen L, Chen J, Ren J, et al. Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J Agric Food Chem. 2011;59:2600–2609.10.1021/jf103771x
- Hu H, Wu J, Li-Chan ECY, et al. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30:647–655.10.1016/j.foodhyd.2012.08.001
- Stathopulos PB, Scholz GA, Hwang YM, et al. Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci. 2004;13:3017–3027.
- Vivian JT, Callis PR. Mechanisms of tryptophan fluorescence shifts in proteins. Biophysical J. 2001;80:2093–2109.10.1016/S0006-3495(01)76183-8