Abstract
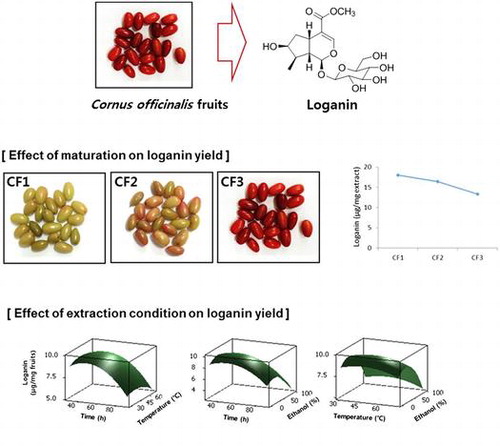
Efficient preparation of loganin from Cornus officinalis fruits was investigated. First, effect of extraction conditions on loganin yield was measured. The loganin content in C. officinalis extract was greatly affected by ethanol concentration and extraction time whereas extraction temperature exerted relatively little effect. Response surface methodology with Box–Behnken design suggested optimized extraction condition for maximum loganin yield as ethanol concentration, 32.0%; temperature 46.2 °C and extraction time, 46.7 min, which yielded 10.4 μg loganin/mg dried fruit. Next, the effect of maturation stage of C. officinalis fruits on loganin content was investigated. The loganin content in the extract of C. officinalis fruits was decreased as the maturation process. The loganin content in the unripe fruits was 18.0 μg/mg extract whereas reduced to 13.3 μg/mg extract for ripe fruits. Taken together, our present study suggested the importance of extraction condition and maturation stages for efficient preparation of loganin from C. officinalis fruits.
Graphical abstract
Importance of extraction condition and maturation stages for efficient preparation of loganin from Cornus officinalis fruits.
Plants including fruits and vegetables nourish people in nutritional values. They also heal the diseases for people in diverse ways. The importance of plants products as raw materials and processed products is countless. However, quality security is sometimes limited due to improper management. Cultivation and post-harvest procedure are essential factors that affect quality assurance. The content of active constituents together with their biological activity of raw materials or extract are highly affected by diverse factors. Extraction conditions such as extraction solvent, extraction time, extraction temperature, and ratio of sample/solvent greatly affect the composition of extract.Citation1–4) The content of active constituents also varies depending on environmental conditions during cultivation. Maturation is one of the main factors that influence the composition as well as content of active constituents.Citation5–7) Therefore, consideration of these factors is required for maximum quality and economic values.
Cornus officinalis, which belongs to Cornaceae, is widely distributed in East Asia including South Korea. The mature fruits of C. officinalis are usually collected in autumn and dried after removal of pits. They have been traditionally used to nourish the liver and kidney and to induce astringent action for loss for body fluid.Citation8) Recently, antioxidant, anti-inflammatory, and hypoglycemic activity of the extract of C. officinalis fruits have been reported.Citation9–11)
Loganin is an iridoid glycoside and known as a representative compound of C. officinalis fruits (Fig. ). Loganin has protective effects against diabetes and related symptoms.Citation12–15) It also showed the beneficial effects on brain functions. It increased cognitive-enhancing activity in amnesic mice and improved neurological function after ischemia.Citation16–18) In addition, anti-melanogenesis, neuroprotective, and anti-inflammatory activity of loganin were also reported.Citation19–22) Due to its diverse activity, C. officinalis fruits and loganin have been developed as health functional food products.
For the development of loganin as functional products, efficient preparation of loganin is required to save cost and time in economic aspects. For this reason, this study was conducted to optimize the extraction condition for maximal yield of loganin using response surface methodology. In addition, due to the importance of maturation in the content of constituents, loganin content in different maturation stages of C. officinalis fruits was also investigated.
Experimental
Plant material
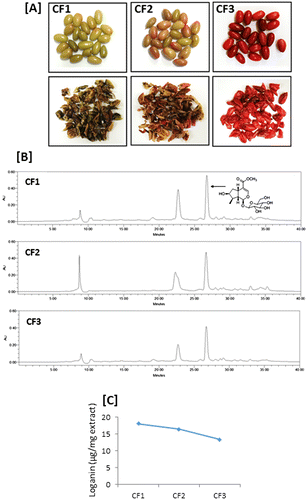
The fruits of C. officinalis were collected from the herb garden at Chungbuk National University in June (CF1, green color), August (CF2, green-to-red color) and October (CF3, red color), 2014 (Fig. (A)). After identification of the fruits by the herbarium of the College of Pharmacy at the Chungbuk National University, the fruits were dried after removal of pits. Voucher specimens for CF1, CF2, and CF3 were deposited in a specimen room of the herbarium.
Measurement of fruit color
Measurement of the color of C. officinalis fruits was carried out using CR-400 colorimeter (Konica minolta, Japan). Values of L*, a*, and b* were used to define the colors. The L* value indicates the lightness (0 for opaque and 100 for transparent or white), a* value indicates redness (+a* for red and −a* for green), and b* value indicates yellowness (+b* for yellow and −b* for blue).Citation23)
Response surface of methodology
Effect of extraction conditions on loganin content of C. officinalis fruits was analyzed using Box–Behnken design (BBD) with three variables and three levels. Extraction time (X1), extraction temperature (X2), and ethanol concentration (X3) were chosen for independent variables, meanwhile loganin content was as dependent response.
Regression analysis was performed according to the experimental data and the mathematical model can be explained by the following equation:
where Y is the response, β0, βi, βii, βij are the coefficients for intercept, linearity, square, and interaction, respectively. The regression equation was evaluated as well-matched form by the lack of fit and coefficient of determination (R2). In addition, analysis of variance (ANOVA) was performed to get p-value which can confirm the factor significant if it is under 0.05. At last, further investigation under optimal condition was performed to confirm that it matched well with predicted value.
Quantitation of loganin content
The loganin content was calculated by HPLC analysis. The loganin was prepared in MeOH at a concentration of 2.0, 1.0, 0.5, 0.25, and 0.1 mg/ml for the preparation of calibration curve of loganin. HPLC analysis was performed using a Waters HPLC system equipped with Waters 600 Q-pumps, a 996 photodiode array detector and Waters Empower software using Phenomenex Gemini-NX 3u C18 110A (150 mm × 10 mm) for quantitation. The mobile phase, consisting of solvent A (Acetonitrile with 0.1% acetic acid) and solvent B (Water with 0.1% acetic acid), was eluted by gradient way with a flow rate of 1.0 ml/min and the procedure was as follows: 10–20% A (0–10 min), 20–40% A (10–20 min), 40–80% A (20–35 min), and 80–10% A (35–37 min). UV detection was set at 237 nm. The linear regression and the coefficient of determination (R2) were calculated on the basis of calibration curve.
Results and discussion
Effect of extraction variables on loganin content
Effect of extraction conditions on loganin content of C. officinalis fruits was analyzed using BBD with three variables and three levels. Extraction time (X1), extraction temperature (X2), and ethanol concentration (X3) were chosen for independent variables, meanwhile loganin content as dependent response. Three levels of these variables were determined as extraction time (X1, 30, 60, and 90 min), extraction temperature (X2, 30, 50, and 70 °C), and ethanol concentration (X3, 0, 50, and 100%) on the basis of preliminary single factor experiment. The variables were coded at three levels (−1, 0 and 1) and complete design was consisted of 15 experimental points including three replication of the center points whose variables were all coded as zero (Table )
Table 1. A BBD for extraction conditions on loganin content.
Multiple regression analysis (Table ) on the experiment data yielded the second-order polynomial regression equations as:
Table 2. Regression coefficients and their significances in the second-order polynomial regression equation for loganin content.
The significance of each coefficient was determined using t-test and p-values as shown in Table . The linear term of extract time (X1, p-value of 0.004) and ethanol concentration (X3, p-value of 0.005) had the significant effect on loganin content, followed by quadratic term of ethanol concentration () and time (X12), whereas linear, quadratic term of temperature and interaction terms of three variables were not significant. The fitness of the predicted model was supported by F-value of 8.95 and p-value of 0.013. The values of coefficient determination (R2) and the adjusted coefficient determination (adj. R2) of predicted model in this response were 0.942 and 0.836, respectively, suggesting that regression equation can explain the observed value with the high degree. In addition, p-value of lack-of-fit was 0.145, which is insignificant relative to the pure errors. Overall, ANOVA supported the good fit of experimental and predicted values and availability of this polynomial model for further optimization.
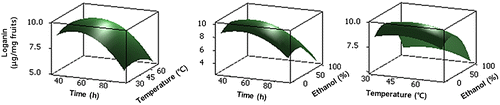
Relationship between every three variables in loganin content was shown in three-dimensional response surface based on regression equations (Fig. ). Consistent with regression coefficient and their significances, ethanol concentration and extraction time clearly affected the loganin yield. The loganin yield was increased with increasing extraction time and ethanol concentration at a certain level, but decrease thereafter. However, loganin yield was slightly affected by extraction temperature.
Taken together, response surface analysis as well as statistical analysis showed that loganin content was greatly changed following ethanol concentration and extraction time, whereas temperature exerted relatively little effect on it.
On the basis of our results, extraction condition for maximum loganin content was optimized as extraction time, 46.7 min; temperature 46.2 °C and ethanol concentration, 32.0% by response surface methodology. To confirm the optimal condition, further investigation was performed and the loganin content was found to be 10.4 μg/mg dried fruit, which was well matched with the predicted value of 9.9 μg/mg dried fruit (Table ).
Table 3. Predicted and observed values of loganin content under optimized condition.
Effect of maturation stages on loganin content
The active constituents of fruits were contained differently depending on various environmental factors. Therefore, the effect of maturity of C. officinalis fruits on the content of loganin was investigated. The fruits of C. officinalis were collected at different maturation stages. They were divided into three groups by their color appearance such as CF1 (green color), CF2 (green-to-red color), and CF3 (red color), which was confirmed by color-difference meter (Table ). The a* value which determines red (+) or green (−) were significantly different among three groups, whereas little differences of the L* and b* values.
Table 4. Color measurement of C. officinalis fruits of three different maturation stages.
HPLC analysis of loganin content in each group suggested that the loganin content was decreased as the maturation of C. officinalis fruits processes (Fig. ) The loganin content was the highest in CF1 (18.0 μg/mg extract), followed by CF2 (16.4 μg/mg extract) and CF3 (13.3 μg/mg extract). Maturation of fruits is usually accompanied by the accumulation of sugars and anthocyanins.Citation24–26) In our present study, the color of C. officinalis fruits turned red in CF3 by the accumulation of anthocyanin, which was confirmed by color-difference meter. In addition, sugar content of CF3 was higher compared to CF1 and CF2 (data not shown). Therefore, decrease in loganin content in ripe fruits might be explained, in some parts, by increase in sugar and anthocyanin amount in the extract of C. officinalis fruits. Collectively, high concentration of loganin can be prepared from the extract of unripe fruits of C. officinalis.
Author contributions
Jong Hoon Ahn, Eun Jin Mo, and Mi Kyeong Lee conceived and designed the experiments. Jong Hoon Ahn, Eun Jin Mo, Yang Hee Jo, and Seon Beom Kim performed the experiments. Jong Hoon Ahn, Eun Jin Mo, Bang Yeon Hwang, and Mi Kyeong Lee analyzed data. Jong Hoon Ahn and Mi Kyeong Lee wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the National Research Foundation of Korea under Basic Science Research Program [grant number 2015R1D1A1A09058498].
References
- Herzi N, Bouajila J, Camy S, et al. Comparison of different methods for extraction from Tetraclinis articulate: yield, chemical composition and antioxidant activity. Food Chem. 2013;141:3537–3545.10.1016/j.foodchem.2013.06.065
- Xu Q, Shen Y, Wang H, et al. Application of response surface methodology to optimize extraction of flavonoids from fructus sophorae. Food Chem. 2013;138:2122–2129.10.1016/j.foodchem.2012.11.099
- Jeong JY, Jo YH, Kim SB, et al. Pancreatic lipase inhibitory constituents from Morus alba leaves and optimization for extraction conditions. Bioorg Med Chem Lett. 2015;25:2269–2274.10.1016/j.bmcl.2015.04.045
- Jeong JY, Jo YH, Lee KY, et al. Optimization of pancreatic lipase inhibition by Cudrania tricuspidata fruits using response surface methodology. Bioorg Med Chem Lett. 2014;24:2329–2333.10.1016/j.bmcl.2014.03.067
- Wang SY, Chen CT, Wang CY, et al. Resveratrol content in strawberry fruit is affected by preharvest conditions. J Agric Food Chem. 2007;55:8269–8274.10.1021/jf071749x
- Bizjak J, Mikulic-Petkovsek M, Stampar F, et al. Changes in primary metabolites and polyphenols in the peel of ‘Bareburn’ apples (Malus domestica Borkh.) during advanced maturation. J Agric Food Chem. 2013;61:10283–10292.10.1021/jf403064p
- Pensec F, Pączkowski C, Grabarczyk M, et al. Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the Upper Rhine Valley. J Agric Food Chem. 2014;62:7998–8007.10.1021/jf502033s
- Wu JN. Chinese Materia Medica. New York (NY): Oxford University Press; 2005. p. 218–219.
- Sung YH, Chang HK, Kim S, et al. Anti-inflammatory and analgesic effects of the aqueous extract of Corni Fructus in murine RAW 264.7 macrophage cells. J Med Food. 2009;12:788–795.
- He K, Song S, Zou Z, et al. The hypoglycemic and synergistic effect of loganin, morroniside, and ursolic acid isolated from the fruits of Cornus officinalis. Phytother Res. 2016;30:283–291.10.1002/ptr.v30.2
- Hwang KA, Hwang YJ, Song J, et al. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officinalis on RAW 264.7 cells. BMC Complement Altern Med. 2016;16:196.
- Yokozawa T, Kang KS, Park CH, et al. Bioactive constituents of Corni Fructus: The therapeutic use of morroniside, loganin, and 7-O-galloyl-D-sedoheptulose as renoprotective agent in type 2 diabetes. Drug Discovery Ther. 2010;4:223–234.
- Jiang WL, Zhang SP, Hou J, et al. Effect of loganin on experimental diabetic nephropathy. Phytomedicine. 2012;19:217–222.10.1016/j.phymed.2011.08.064
- Ma W, Wang KJ, Cheng CS, et al. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J Ethnopharmacol. 2014;153:840–845.10.1016/j.jep.2014.03.051
- Liu K, Xu H, Lv G, et al. Loganin attenuates diabetic nephropathy in C57BL/6J mice with diabetes induced by streptozotocin and fed with diets containing high level of advanced glycation end products. Life Sci. 2015;123:78–85.10.1016/j.lfs.2014.12.028
- Lee KY, Sung SH, Kim SH, et al. Cognitive-enhancing activity of loganin isolated from Cornus officinalis in scopolamine-induced amnesic mice. Arch Pharm Res. 2009;32:677–683.10.1007/s12272-009-1505-6
- Yao RQ, Zhang L, Wang W, et al. Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res Bull. 2009;79:69–76.10.1016/j.brainresbull.2008.12.010
- Kwon SH, Kim HC, Lee SY, et al. Loganin improves learning and memory impairments induced by scopolamine in mice. Eur J Pharmacol. 2009;619:44–49.10.1016/j.ejphar.2009.06.062
- Nawa Y, Endo J, Ohta T. The inhibitory effect of the components of Cornus officinalis on melanogenesis. J Cosmet Sci. 2007;58:505–517.
- Youn K, Jeong WS, Jun M. β-Secretase (BACE1) inhibitory property of loganin isolated from Corni fructus. Nat Prod Res. 2013;27:1471–1474.10.1080/14786419.2012.718774
- Kim H, Youn K, Ahn MR, et al. Neuroprotective effect of loganin against Aβ25-35-induced injury via the NF-κB-dependent signaling pathway in PC12 cells. Food Funct. 2015;6:1108–1116.10.1039/C5FO00055F
- Kim MJ, Bae GS, Jo IJ, et al. Loganin protects against pancreatitis by inhibiting NF-κB activation. Eur J Pharmacol. 2015;765:541–550.10.1016/j.ejphar.2015.09.019
- Serradilla MJ, Lozano M, Bernalte MJ, et al. Physicochemical and bioactive properties evolution during ripening of ‘Ambrunes’ sweet cherry cultivar. Food Sci Technol. 2011;44:199–205.
- Liang Z, Sang M, Fan P, et al. Changes of polyphenols, sugars, and organic acid in 5 Vitis genotypes during berry ripening. J Food Sci. 2011;76:C1231–C1238.10.1111/jfds.2011.76.issue-9
- Li WQ, Hu QP, Xu JG. Changes in physicochemical characteristics and free amino acids of hawthorn (Crataegus pinnatifida) fruits during maturation. Food Chem. 2015;175:50–56.10.1016/j.foodchem.2014.11.125
- Almeida ML, Freitas WE, de Morais PL, et al. Bioactive compounds and antioxidant potential fruits of Ximenia americana L. Food Chem. 2016;192:1078–1082.10.1016/j.foodchem.2015.07.129