Abstract
Atherosclerosis is one of the diseases related to metabolic syndrome which is caused by obesity. Previous reports have shown that green tea and its components have anti-obesity effect. We examined whether catechins and caffeine can prevent the development of atherosclerosis by oral administration, singly or in combination to the atherosclerosis model mice. Results demonstrated that the number of atherosclerotic regions in the aorta was significantly reduced by the combined treatment, and the atherosclerotic area was also improved. Serum HDL-C increased by caffeine single treatment, but no effect on the TG and TC by any treatments. Moreover, ECG illuviated to atheromatous lesions in aorta and the illuviation was enhanced by caffeine. The mRNA expression levels of LOX-1 and TNF-α showed a tendency to suppress by the combined treatment. These results indicated that the combined administration of catechins and caffeine has the inhibitory effect on the development of atherosclerosis in mice.
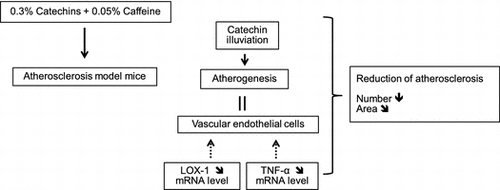
Our research clearly demonstrated that the combined treatment of catechins and caffeine showed the inhibitory effect on the development of atherosclerosis in mice.
Key words:
Recently, metabolic syndrome often caused by obesity has become a social problem in developed countries. Particularly, atherosclerosis is one of the most serious diseases related to metabolic syndrome and can lead to death. The development of atherosclerosis is strongly related to physiological factors such as the production and secretion of inflammatory cytokines, the adhesion of monocytes to vascular endothelial cells, the invasion of monocytes into the vascular endothelial cells, the phagocytosis of oxidized low-density lipoprotein (oxidized LDL) by macrophages, and the formation of foam cells.
On the other hand, green tea is one of the most popular beverages in the world. Previous reports have demonstrated that green tea has an anti-obesity action. Green tea has a lot of catechins and several other trace components having physiological functions like caffeine, theanine, and vitamins. Among green tea components, green tea catechins have many physiological effects such as anti-angiogenesis,Citation1) anti-oxidation,Citation2) cholesterol blood disease onset inhibition,Citation3) anti-cancer,Citation4) and apoptosis induction.Citation5) In particular, the formation of oxidized LDL is inhibited by catechins through their strong antioxidant action.Citation6) Thus, the anti-inflammatory action of green tea polyphenols is involved in the suppression of atherogenesis. Moreover, it has been reported that administration of green tea catechins with caffeine ameliorated plasma lipid levels and suppressed the development of atherosclerosis in ApoE-null mice through antioxidant activity.Citation7) However, the paper showed no data about the accurate concentrations of catechins and caffeine and the effects of a single treatment with them. Moreover, there were no changes in the cholesterol levels and the inhibitory mechanism is still unknown. Furthermore, Yang et al.,Citation8) demonstrated that mRNA expression of tumor necrosis factor-α (TNF-α) in a macrophage cell line was suppressed when the cells were cultured in medium with green tea polyphenols. This result indicated that the reduction of TNF-α in the cells might play an important role if the development and the progression of atherosclerosis are suppressed by green tea polyphenols.
Meanwhile, green tea also contains caffeine, which has many physiological effects, such as a smooth muscle relaxant effect; cardiac, diuretic, vasodilator actions; and central stimulation and metabolic promoting actions.Citation9) So far, excessive consumption of coffee or caffeine is a risk factor for lifestyle-related diseases including osteoporosis and accounts for heart diseases by increasing cholesterol level.Citation10,11) In contrast, Yukawa et al.,Citation12) reported that an adequate dose of coffee significantly reduced blood LDL cholesterol and reduced the susceptibility of oxidized LDL, which promotes atherogenesis.
The main cause of atherosclerosis is obesity. Zheng et al.,Citation13) reported that green tea has a strong anti-obesity action. Moreover, among several green tea components, a combination of catechins and caffeine brought about the same anti-obesity effect as green tea in mice. Furthermore, as the mechanism of this action, these components lowered the concentrations of several lipids in serum and the liver through the suppression of lipid synthesis and promotion of lipid oxidation in the liver. Thus, it was supposed that joint administration of catechins and caffeine might strongly suppress atherogenesis compared to a single administration of them. Therefore, in this research, the effects of catechins and caffeine on the development of atherosclerosis were examined in model mice.
Materials and methods
Animals
We used femaleC57BL/6.KOR/Stm-Apoeshl, (B6.KOR-Apoeshl) mice, which were established by Matsushima et al.,Citation14) as atherosclerosis model mice. Four-week-old B6.KOR-Apoeshl mice of about 10–15 g, maintained by brother–sister mating in the animal room in Shizuoka university, were used in this research. Each mouse was kept in the following conditions: 24 ± 2 °C room temperature, 50 ± 10% humidity, 12-h light period, 12-h dark period (8:00 am lighting, 8:00 pm off). All animal care and experiments were conducted in accordance with the guidelines of the Science Council of Japan, the Japanese Association for Laboratory Animal Science, and protocols were approved by the animal care and use committee at Shizuoka University.
Diets
Decaffeinated crude catechin extract (Polyphenon 70S, Mitsui Norin Co., Ltd., Japan; Table ) and caffeine (purity 98.5%, Wako Pure Chemical Industries, Ltd., Japan) were used for the feeding experiment. In accordance with the previous report by Zheng et al.,Citation13) 0.3% catechins and 0.05% caffeine were mixed singly or in combination with commercial mouse powder diet (MF powder, Oriental Yeast Co., Ltd., Japan) and used as the experimental diets.
Table 1. Composition of Polyphenon 70S.
Feeding experiment and measurement of body and organ weights
The following four experimental groups were made for the feeding experiments: 0.3% catechins, 0.05% caffeine, 0.3% catechins + 0.05% caffeine, and control. Ten female mice were used for each group, and were fed the diets along with water ad libitum during the administration period for 12 weeks. We maintained five mice in each cage. Thus, male mice were not used for this research as to avoid the individual specificity arising from social stress by group breeding hierarchy. During the period, body weight gain was measured once a week and feed intake was measured three times a week. Feed intake was measured using a special feeder for measurement of feed consumption (Oriental Yeast Co., Ltd.), and the amount of feed intake per animal was calculated by the total feed intake from the beginning to the end of 12 weeks.
After the feeding period, each mouse was fasted for 12 h. Then, each was killed by over-anesthesia with diethyl ether. The blood collected from the heart and the serum was centrifuged (15 min, 800 × g), collected, and stored in a freezer at −80 °C until analysis. After blood collection, the remaining blood in vivo was removed by ischemic perfusion with phosphate buffered saline (PBS). Livers were frozen in dry ice immediately after weighing and then kept in a freezer at −80 °C until analysis.
Preparation of the aorta
The aortic tree of mice was prepared as previously described by Tangirala et al.,Citation15) with some modification. Briefly, under a stereoscopic microscope, the fat and other tissues around the aorta were removed. The bifurcation of the right common carotid artery and the right subclavian artery in the brachiocephalic artery was excised about 1 mm from the aorta. The left subclavian artery and common carotid artery were also cut about 1 mm from the aorta. Then, the aortic tree from the heart to the diaphragm was exposed after the minor branching arteries were cut off. The aorta from the heart to the diaphragm was dissected, opened, pinned on a silicone bed by micro-steel pins (Fine Science tools, USA), and immersed in PBS. Then, the aorta was fixed with 4% paraformaldehyde for 10 min and washed three times in PBS for 5 min with gentle shaking.
Lipid-staining and analysis of atheromatous lesions in the aorta
The Nile red staining of atheromatous lesions in the aorta was performed by the method of Klinkner et al.Citation16). The fixed aorta was stained by Nile red solution for 30 min. After the staining, the aorta was washed three times for 3 min in PBS. Then, the aorta was mounted on a slide with non-fluorescent mounting solution (Immu-mount, Thermo Scientific, USA). The stained aorta was observed and photographed using a fluorescence stereomicroscope (Olympus Co., Japan). The picture image was record as an electronic file by computer and the number of lipid staining regions and the ratio of the positive staining area in the aorta were analyzed by image analyzing software (Photoshop, Adobe, USA and Easy Access, AD Science Co., Japan).
Measurement of lipid, lipid peroxide, and TNF-α levels in serum
The concentrations of triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) were measured in the serum of each mouse using the following test kits: Triglyceride E-Test Wako, Cholesterol E-Test Wako and HDL-cholesterol E-Test Wako (Wako Pure Chemical Industries, Ltd., Japan). Lipid peroxide in the serum was measured using the TBARS Assay Kit (Cayman Chemical Company).
RNA preparation and semi-quantitative RT-PCR
In order to elucidate the inhibitory mechanism of atherogenesis by catechins and caffeine, mRNA expression levels were measured for vascular cell adhesion molecule 1 (VCAM-1), an adhesion molecule; TNF-α, an inflammatory cytokine related to the development and the progression of atherosclerosis; monocyte chemotactic protein 1 (MCP-1), a chemokine that is involved in the attraction of macrophages; and lectin-like oxidized LDL receptor 1 (LOX-1), a receptor of oxidized cholesterol in macrophages. In addition, another set of feeding experiments was done and the aorta was collected by the same protocols as mentioned above. Aortas were dissected from each animal and stored at −80 °C in RNA later solution (Applied Biosystems, New Jersey, USA) for subsequent RNA isolation. Total RNA was isolated with an RNeasy Mini Kit(QIAGEN, Germany)according to the manufacturer’s instructions, followed by deoxyribonuclease digestion.
In order to prevent the contamination of recovered tRNA solution from genomic DNA, an RNase-free DNase set (QIAGEN) was used. After the removal of genomic DNA, total RNA was purified with a spin column. The extracted RNA was analyzed by reverse transcription polymerase chain reaction (RT-PCR) using the SuperScriptTM First-Strand Synthesis System for RT-PCR (Invitrogen, USA) according to the supplier’s instructions, with the exception of RT, which was performed with oligo d(T)16. Semi-quantitative RT-PCR analysis was used for the comparison of mRNA expression levels of LOX-1, MCP-1, TNF-α, and VCAM-1 in the aorta from each experimental group. PCR was performed in a thermal cycler (PC-818A, ASTEC, Japan) according to the following specifications, and each primer for RT-PCR was shown as follows:
| • | LOX-1Citation17) | ||||
94 °C for 30 s to denature the DNA, 60 °C for 30 s to allow for primer annealing, and 72 °C for 30 s for extension, all of which were performed for 34 cycles (Forward: 5′-AGGTCCTTGTCCACAAGACTGG-3′; Reserve: 5′-ACGCCCCTGGTCTTAAAGAATTG-3′).
| • | MCP-1Citation18) | ||||
94 °C for 30 s to denature the DNA, 60 °C for 30 s to allow for primer annealing, and 70 °C for 30 s for extension, all of which were performed for 30 cycles (Forward: 5′-CTTCTGGGCCTGCTGTTCA-3′; Reverse: 5′-CCAGVVTACTCATTGGGATCA-3′).
| • | TNF-αCitation19) | ||||
94 °C for 15 s to denature the DNA, 58 °C for 30 s to allow for primer annealing, and 72 °C for 30 s for extension, all of which were performed for 31 cycles (Forward: 5′-AAATGGGCTTTCCGAATTCA-3′; Reverse: 5′-CAGGGAAGAATCGGAAAGGT-3′).
| • | VCAM-1Citation20) | ||||
94 °C for 30 s to denature the DNA, 55 °C for 30 s to allow for primer annealing, and 72 °C for 30 s for extension, all of which were performed for 29 cycles (Forward: 5′-AAGCAGAGACTTGAAATGCC-3′; Reverse: 5′-CCCTTGAACAGATCAATCTCC-3′).
| • | β-actinCitation21) | ||||
94 °C for 30 s to denature the DNA, 60 °C for 30 s to allow for primer annealing, and 72 °C for 1 min for extension, all of which were performed for 23 cycles (Forward: 5′-TCGTGCGTGACATCAAAGAG-3′; Reverse: 5′-TCTCCTTCTGCATCCTGTCA-3′).
The amplified DNA was subjected to electrophoresis on a 1.5% agarose gel, and stained with ethidium bromide. The intensity of each PCR product for each gene of interest was measured with image J software, and the resulting densitometric value was compared to those of β-actin, the internal standard.
Detection of catechins in atheromatous lesions in the aorta
Kawai et al.,Citation22) reported that epicatechin gallate (ECG), one of the green tea catechins, binds to atheromatous lesions in the human aorta, as revealed by immunohistochemical staining using ECG antibody. Thus, another set of feeding experiments: 0.3% catechins, 0.3% catechins + 0.05% caffeine, and control, was done for 2 weeks, and the aortas were collected using the same protocol as mentioned above. The collected aorta was opened, pinned, washed in PBS for 5 min after fixation, and incubated for 1 h with blocking solution (0.3% H2O2/PBS) containing 1% bovine serum albumin (BSA), 1% normal rat serum, and 0.1% Tween 20. After incubation, the primary antibody, rabbit anti-mouse ECG antibody produced by Kawai et al., was reacted overnight at 4 °C. After the reaction, the secondary reaction was done using the Histofine Stain Kit for mice (Nichirei Co., Japan). After immunostaining, samples were washed three times in PBS for 5 min. Stained samples were then mounted on a slide, observed by stereomicroscope, and photographed (Leica Microsystems Co., Germany). After immunostaining of ECG, each aorta was also stained by Nile red to ascertain the atheromatous lesions in the aorta. All the pictures were record as an electronic file by computer.
Statistical analysis
Two-way ANOVA was used for the statistical analysis of all the data. Tukey’s test was performed when the significant value was observed by two-way ANOVA. Data are presented as means ± SE, values of p < 0.05 were considered significant.
Results
Body weight gain and feed intake
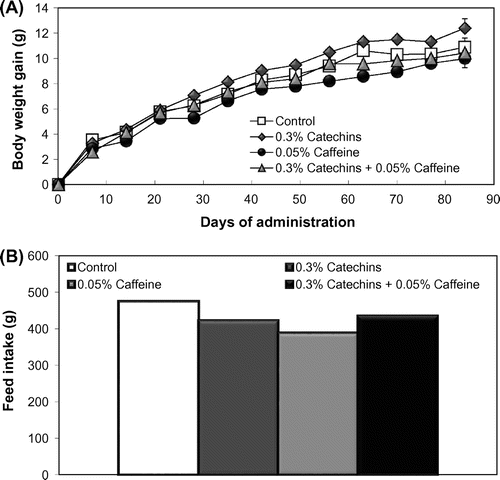
Body weight gain and feed intake during the administration period of each group are shown in Fig. . Body weight gain per mouse during the administration period was not statistically different between the control and the experimental groups. The total feed intake also showed no difference but not analyzed statistically because of group breeding using two cages in each experimental group.
Lipid staining of the aorta
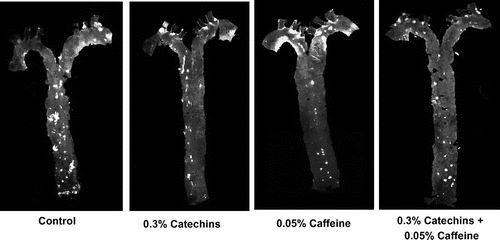
After the feeding period, each aorta was collected, spread on a slide with the inside facing outwards, and stained by Nile red. As shown in Fig. , the number and the total area of positive staining regions in the aortas from mice treated with both catechins and caffeine were visibly smaller than in other groups. However, the intensity of staining in the single treated mice was not visibly different.
Number and area of atheromatous lesions in the aorta
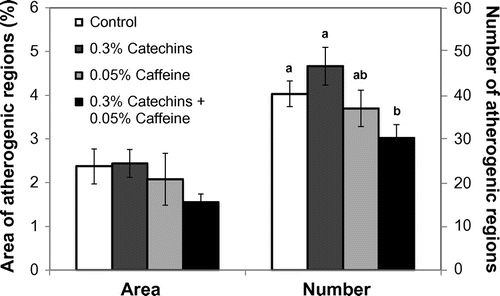
The intensity of positive staining of atheromatous lesions was visibly different between the catechins + caffeine group and other groups. Thus, the number of lesions and the positive area were analyzed. As a result, the number of atheromatous lesions in the aorta from the catechins + caffeine group was significantly reduced compared to the control group and the catechin single treatment group (Fig. ). Moreover, the lesion area of catechins + caffeine group was approximately 36% less than that of the control, but no significant difference between them was observed (Fig. ).
Lipid levels in serum
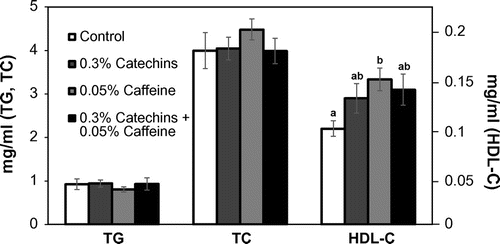
As Fig. shows, there were no effects on the serum levels of TG and TC by the catechins and/or caffeine treatment. The HDL-C level in the serum was significantly increased by caffeine single treatment compared to the control (Fig. ). However, the HDL-C level in the mice was still very low due to a gene mutation for the apoE protein.
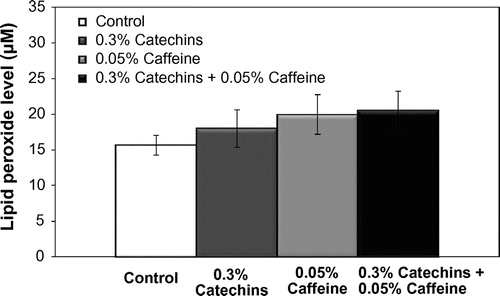
Lipid peroxide level in the serum
The levels of lipid peroxide in the serum are shown in Fig. . The level of lipid peroxide was not affected by caffeine and/or catechins (Fig. ).
Semi-quantitative RT-PCR
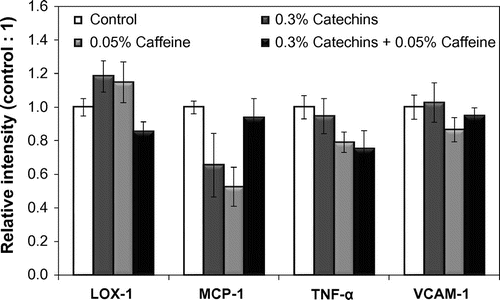
The mRNA expression levels of LOX-1, MCP-1, TNF-α, and VCAM-1, which have been reported to relate to the development of arteriosclerosis in the mouse aorta, were analyzed.Citation23)
A decreasing trend of LOX-1 and TNF-α expression in the aorta was shown in the catechins + caffeine and the caffeine groups, respectively (Fig. ). The expression of MCP-1 also showed a tendency to decrease by the caffeine treatment. There were no significant differences among each group in VCAM-1 expression.
Detection of catechin in atheromatous lesions in the aorta
Fig. shows the localization of epicatechin gallate (ECG; Fig. (a), (c), (e), (g)) and the accumulation of lipid atheromatous lesions (Fig. (b), (d), (f), (h)) in the aorta after the treatment with catechins and/or caffeine for 2 weeks. A strong positive reaction of ECG was observed in atheromatous lesions in the aorta from the catechins + caffeine-treated mice following immunohistochemical staining with ECG antibodies (Fig. (g)). Positive staining was also detected in the lesions from catechin-treated mice (Fig. (e)). The positive intensity in catechins + caffeine-treated mice was stronger than in catechins-treated mice.
Fig. 7. Localization of epicatechin gallate (ECG) in atherogenic regions (Fig. (a), (c), (e), (g)) and detection of lipid accumulation in atheromatous lesions (Fig. (b), (d), (f), (h)) after the treatment with catechins and/or caffeine. Immunostaining with ECG antibody of negative staining (Fig. (a)), normal serum was used as the primary antibody), Control (Fig. (c)), 0.3% catechins (Fig. (e)) and 0.3% catechins + 0.05% caffeine (Fig. (g)). Black arrows indicate the positive staining regions and dark grey areas surrounding the holes of narrow branched arteries in the aorta show ECG positive staining. After immunostaining, each aorta was also stained by Nile red for the detection of lipid accumulation in atheromatous lesions (Fig. (b), (d), (f), (h)). White arrows indicate the positive staining area of lipids in the aorta. White areas surrounding the holes of narrow branched arteries in the aorta show lipid accumulation.
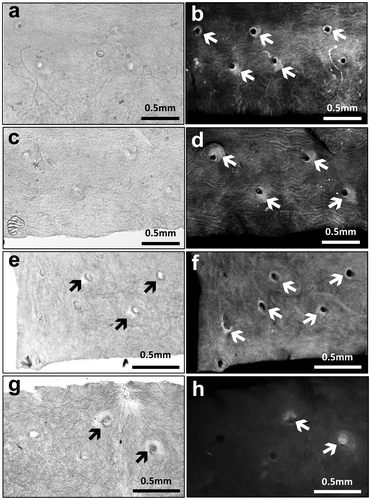
After lipid staining, ECG positive regions in the aorta coincided with the positive lipid staining regions. Moreover, the absence of positive staining showed a decrease in lipid accumulation in both the single administration and the combined administration, compared to the control and the negative control (Fig. (b), (d), (f), (h)). However, there was hardly any difference in staining between the single administration and the combination administration.
Discussion
In this study, it was clearly demonstrated that the number of atheromatous lesions in the mouse aorta was significantly suppressed and the area of atheromatous lesions was improved by the combined administration of catechins and caffeine. However, single administration of each had no significant effect. Therefore, the combined administration of catechins and caffeine might have an inhibitory effect on both the development and the progression of atherosclerosis. Previously, it was shown that arteriosclerosis was inhibited by the administration of green tea catechins with caffeine in drinking water.Citation7) However, there were no data about the inhibiting mechanism in the paper. Thus, we also analyzed several biomarkers which are involved in the atherogenesis in serum and aorta in mice.
It is known that the first stage of atherosclerosis is caused by inflammation in the curved and branched regions in the aorta, which sustain physical blood pressure and shear stress. TNF-α is an inflammatory cytokine that is expressed in inflamed vessels and is released in a large amount during the process of atherogenesis. It has been reported that TNF-α in vascular endothelial cells promotes endothelial damage.Citation1) Moreover, a significant reduction in atherosclerotic sites was observed in mice deficient of TNF-α. Thus, it was considered that TNF-α is strongly involved in the development of atherosclerosis.Citation3) In this research, mRNA expression of TNF-α in the aorta in catechins + caffeine administered group showed decreasing trend, even though the serum TNF-α was not suppressed by the treatment. Therefore, it would appear that these administrations would locally suppress TNF-α expression in atheromatous lesions in the aorta and would suppress the following attraction of monocytes, which are involved in atherogenesis of vascular endothelial cells by inflammation.
LOX-1 is one of the receptors in vascular endothelial cells and macrophages for oxidized cholesterol, which is involved in atherogenesis.Citation24) The uptake of oxidized LDL by macrophages via LOX-1 leads to the formation of foam cells and promotes the formation of fatty streaks and the development of plaques in atherogenic regions.Citation1) Therefore, inhibition of LOX-1 expression in the aorta can prevent atherosclerosis. In this research, mRNA expression levels of LOX-1 in aorta after the treatment of diets with catechins and caffeine showed a tendency to decrease. This suggests that catechins and caffeine might suppress LOX-1 expression in atherogenic regions and, as a result, inhibit both the intake of oxidized cholesterol by macrophages and vascular endothelial cells and the following alteration from macrophages to foam cells. Moreover, it is known that LOX-1 expression in macrophage is promoted by TNF-α.Citation25) Therefore, the suppression of LOX-1 expression might be caused by suppression of TNF-α by catechins and caffeine.
TNF-α-induced mRNA expression of VCAM-1 in endothelial cells increases in atherosclerotic sites in mouse arteries.Citation4) Sung et al.,Citation26) reported that serum VCAM-1 level was significantly decreased after ingestion of green tea for 4 weeks. Moreover, EGCG suppressed VCAM-1 expression in human vascular endothelial cells stimulated by TNF-α in vitro.Citation27) Therefore, it was considered that the expression of these adhesion molecules is decreased by catechins and caffeine. However, there was no change in mRNA expression of VCAM-1 in aorta from catechins and catechins + caffeine groups.
It was reported that ECG was brought in macrophages and form cells and accumulates in human atherosclerotic lesions.Citation22) ECG was detected in the atheromatous lesions in aorta even in mice, and the intensity was stronger in the aorta from the catechins + caffeine-treated mice compared to the catechins-treated mice (Fig. ). This clarified that catechins, including ECG, are taken into the regions even in mice, and caffeine might have promoted the uptake. Moreover, after their uptake, catechins might be locally responsible for the antioxidant action of LDL cholesterol and for suppressing the expression of several genes that are induced by inflammation in the mouse aorta. We could not obtain antibodies to other catechins than ECG. Therefore, other catechins also might accumulate in the atheromatous lesions and be involved in the suppression of atherogenesis.
In this research, we used Polyphenon 70S as the crude catechin. Polyphenon 70S has other contents except for catechins at 31.2%. However, the details of the contents have not been analyzed. Polyphenon 70S was mainly made by extraction using ethyl acetate. Therefore, it was supposed that there are no specific and concentrated constituents except for catechins. However, it could not be denied completely that the other components might affect the results of this research.
In conclusion, this research elucidated that the combined administration of catechins and caffeine in mice may be involved in the suppression of vascular endothelial inflammation and the following expression of TNF-α in atherogenic regions and inhibition of oxidized LDL cholesterol uptake through the suppression of LOX-1 in macrophages. Then, the development and the progression of atherosclerosis are inhibited by these suppressive actions. In this research, the feeding experiment was started before the atherosclerosis development in mice. However, usually, detection of human atherosclerosis occurs after the formation of atheromatous lesions. Thus, research into the effects of catechins and caffeine after the development of lesions is ongoing.
Author contributions
K. Sayama, L. Liu, I. Nagai, and Y. Gao designed and performed the research, and analyzed the data. Y. Matsushima and Y. Kawai provided some materials and gave the advice of the research. K. Sayama and L. Liu wrote the manuscript. K. Sayama had the primary responsibility for the final content. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Mitsui Norin Co., Ltd.
Acknowledgment
Authors appreciate Mitsui Norin Co., Ltd. for providing research foundation.
Notes
Abbreviations: TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; ECG, epicatechin gallate; TNF-α, tumor necrosis factor α; LOX-1, lectin-like oxidized LDL receptor-1; MCP-1, monocyte chemotactic protein-1; VCAM-1, vascular cell adhesion molecule 1.
References
- Xu H, Lui WT, Chu CY, et al. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum Reprod. 2009;24:608–618.
- Haque AM, Hashimoto M, Katakura M, et al. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr. 2006;136:1043–1047.
- Muramatsu K, Fukuyo M, Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J Nutr Sci Vitaminol (Tokyo). 1986;32:613–622.10.3177/jnsv.32.613
- Roomi MW, Ivanov V, Kalinovsky T, et al. Antitumor effect of ascorbic acid, lysine, proline, arginine, and green tea extract on bladder cancer cell line T-24. Int J Urol. 2006;13:415–419.10.1111/j.1442-2042.2006.01309.x
- Nakazato T, Ito K, Ikeda Y, et al. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin Cancer Res. 2005;11:6040–6049.10.1158/1078-0432.CCR-04-2273
- Ishikawa T, Suzukawa M, Ito T, et al. Effect of tea flavonoid supplementation on the susceptibility of low-density lipoprotein to oxidative modification. Am J Clin Nutr. 1997;66:261–266.
- Miura Y, Chiba T, Tomita I, et al. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001;131:27–32.
- Yang F, De Villiers WJ, McClain CJ, et al. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998;128:2334–2340.
- Heckman MA, Weil J, Mejia D. Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci. 2010;75:R77–87.10.1111/jfds.2010.75.issue-3
- Kamagata-Kiyoura Y, Ohta M, Saltzman MJ, et al. Combined effects of caffeine and prostaglandin E2 on the proliferation of osteoblast-like cells (UMR106-01). J Periodontol. 1999;70:283–288.10.1902/jop.1999.70.3.283
- Sesso HD, Gaziano JM, Buring JE, et al. Coffee and tea intake and the risk of myocardial infarction. Am J Epidemiol. 1999;149:162–167.10.1093/oxfordjournals.aje.a009782
- Yukawa GS, Mune M, Otani H, et al. Effects of coffee consumption on oxidative susceptibility of low-density lipoproteins and serum lipid levels in humans. Biochemistry (Mosc). 2004;69:70–74.10.1023/B:BIRY.0000016354.05438.0f
- Zheng G, Sayama K, Okubo T, et al. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo. 2004;18:55–62.
- Matsushima Y, Sakurai T, Ohoka A, et al. Four strains of spontaneously hyperlipidmic (SHL) mice: phenotypic distinctions determined by genetic background. J Atheroscler Thromb. 2001;8:71–79.10.5551/jat1994.8.71
- Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–2328.
- Klinkner AM, Bugelski PJ, Waites CR, et al. A Novel technique for mapping the lipid composition of atherosclerotic fatty streaks by en face fluorescence microscopy. J Histochem Cytochem. 1997;45:743–753.10.1177/002215549704500513
- Suga M, Sawamura T, Nakatani T, et al. Expression of lectin-like oxidized low-density lipoprotein receptor-1 in allografted hearts. Transplant Proc. 2004;36:2440–2442.10.1016/j.transproceed.2004.06.036
- Furnkranz A, Schober A, Bochkov VN, et al. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol. 2005;25:633–638.10.1161/01.ATV.0000153106.03644.a0
- Kim W, Kim J, Jung D, et al. Induction of lethal graft-vs.-host disease by anti-CD137 monoclonal antibody in mice prone to chronic graft-vs.-host disease. Biol Blood Marrow Transplant. 2009;15:306–314.10.1016/j.bbmt.2008.11.035
- Petersen EJ, Miyoshi T, Yuan Z, et al. siRNA silencing reveals role of vascular cell adhesion molecule-1 in vascular smooth muscle cell migration. Atherosclerosis. 2008;198:301–306.10.1016/j.atherosclerosis.2007.10.015
- Yamashita H, Abe M, Watanabe K, et al. Vasohibin prevents arterial neointimal formation through angiogenesis inhibition. Biochem Biophys Res Commun. 2006;345:919–925.10.1016/j.bbrc.2006.04.176
- Kawai Y, Tanaka H, Murota K, et al. (–)-Epicatechin gallate accumulates in foamy macrophages in human atherosclerotic aorta: implication in the anti-atherosclerotic actions of tea catechins. Biochem Biophys Res Commun. 2008;374:527–532.10.1016/j.bbrc.2008.07.086
- Gu L, Okada Y, Clinton SK, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281.10.1016/S1097-2765(00)80139-2
- Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013:2013. DOI:10.1155/2013/152786. Epub 2013.
- Moriwaki H, Kume N, Kataoka H, et al. Expression of lectin-like oxidized low density lipoprotein receptor-1 in human and murine macrophages: upregulated expression by TNF-α. FEBS Lett. 1998;440:29–32.10.1016/S0014-5793(98)01414-8
- Sung H, Min WK, Lee W, et al. The effects of green tea ingestion over four weeks on atherosclerotic markers. Ann Clin Biochem. 2005;42:292–297.10.1258/0004563054255597
- Yamagata K, Xie Y, Suzuki S, et al. Epigallocatechin-3-gallate inhibits VCAM-1 expression and apoptosis induction associated with LC3 expressions in TNFα-stimulated human endothelial cells. Phytomedicine. 2015;22:431–437.10.1016/j.phymed.2015.01.011