Abstract
The aim of this study is to examine the anti-inflammatory effect of Euphorbia supina (ES) ethanol extract in dextran sulfate sodium (DSS)-induced experimental colitis model. ES was per orally administered at different doses of 4 or 20 mg/kg body weight with 5% DSS in drinking water for 7 days. Twenty mg/kg of ES administration regulated body weight decrease, recovered colon length shortening, and increased disease activity index score and myeloperoxidase level in DSS-induced colitis. Histological features showed that 20 mg/kg of ES administration suppressed edema, mucosal damage, and the loss of crypts induced by DSS. Furthermore, ES suppressed the expressions of COX-2, iNOS, NF-kB, IkBα, pIkBα in colon tissue. These findings demonstrated a possible effect of amelioration of ulcerative colitis and could be clinically applied.
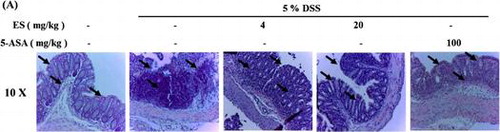
Tissue sections of large intestine showed decrease in crypt epithelium distortion and in mucosal infiltrations, induced by DSS administration. However, these decreases were attenuated by ES oral administration.
Inflammatory bowel disease (IBD) including ulcerative colitis (UC) and Crohn’s disease (CD) is characterized by chronic inflammation and disruption of gut tissue caused by a variety of factors and affects up to 500 people per 100,000 in Western countries.Citation1–7) Although the pathogenesis remains unknown, recent studies suggest that IBD generally has disorders in expression of pro-inflammatory mediators such as reactive oxygen and cytokines induce intestinal mucosal damage.Citation8,9) Fecal transplantation has been recently tried based on the relationship between IBD and human intestinal microbiota, further studies for safety and universal clinical application are needed.Citation12–14) Chemical therapy like 5-aminosalicylic acid (ASA) administration has been considered as an effective treatment, however it causes some side effects like cramp, abdominal pain, diarrhea, and kidney damage.Citation10,11)
Alternatively, herbal therapies for IBD have been intensively studied.Citation15–17) Inhibitory effect of many of herbal materials on IBD was evaluated and some of them showed remarkable output. However, Euphorbia supina (ES), an annual herb which naturally grows in Korea, Japan, and China was little studied as a promising agent for IBD treatment and prevention. ES has been traditionally used for treatment of dysentery and enteritis for thousands of years.Citation18) Considering the usage for gut-related diseases ES might be effective on IBD.
Although the etiology of UC remains unknown, recent studies have suggested that inflammatory response initiated by the reaction between immune system such as interaction between macrophages and dendritic cells and antigens is involved.Citation19) The inflammatory response is induced by activation of macrophages by bacterial products such as lipopolysaccharide (LPS) in UC condition. Activated macrophages release pro-inflammatory such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6. Also, UC patients have been reported that they have high levels of inflammatory cytokines.Citation20) Cytokines are involved in a variety of biological processes, including cell activation, growth, and differentiation, also they are essential to the development of inflammation and immunity.Citation21) The secretions of pro-inflammatory cytokines, such as IL-1, IL-6, IL-8, and TNF-α, are increased and detected in UC and CD patients Citation22) Besides cytokines, many different molecules including adhesion molecules, growth factors, reactive oxygen species (ROS), nitric oxide (NO) are implicated intestinal inflammations.Citation23,24)
In this study, we investigated the inhibitory activities of ES on IBD with dextran sodium sulfate (DSS)-induced colitis model mice. Although ES is known to have various benefits, whether ES might have beneficial effect on UC remains unclear. Therefore, the aim of this study was to determine whether ES could inhibit clinical signs and inflammatory mediators in DSS-induced UC.
Materials and methods
Experimental animal
All experimental protocols (WKU14–74) were approved by the Committee on the Care of laboralab Animal Resources, Wonkwang University and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Male BALB/c mice (7 weeks old, 18–22 g) were purchased from SAMTAKO (Osan, Korea). The mice were group-housed under controlled temperature (25 °C) and photo-period (12–12 h light–dark cycle) conditions and given unrestricted access to standard diet and tap water (or specified drinking solution). The animals were allowed to acclimate to these conditions for at least 7 days before inclusion in experiments.
ES preparation
ES was purchased from Wonpharm (Iksan, Korea). ES was washed twice with distilled water followed by drying. Then, ES was extracted with 70% ethanol at room temperature for 3 days (100 g/L). The solvent ethanol was filtered and allowed to evaporate using a rotary evaporator at a temperature of 40~45 °C. The yield of dried extract was 3.15%. The extract was diluted in 0.9% saline and filtered through a 0.22 μm syringe filter (HUNDAI Micro, Seoul, Korea).
Dextran sulfate sodium-induced colitis
Colitis model was employed as previously described.Citation25) Dextran sulfate sodium (DSS, molecular weight 36–50 kDa) was obtained from MP Biomedicals (Cleveland, OH, USA) and dissolved in distilled water. Colitis was induced by drinking 5% DSS (w/v) for 7 days. Non-colitic control mice were received distilled water during same period. ES extract was suspended in 0.9% NaCl solution and administered p.o. at different doses 4 and 20 mg/kg body weight for 7 days. In this model, behavior, body weight, and stool consistency were checked daily for 7 days. All animals were sacrificed 4 h after last administration.
Disease activity index
A disease activity index (DAI) was determined by scoring the extent of body weight loss, fecal hemoccult positivity or gross bleeding, and stool consistency in accordance with the method described by Murthy et al.Citation26) at sacrifice (Table ). Weight loss was defined as the difference between initial and final weights, and diarrhea as the absence of fecal pellet formation and the presence of continuous fluid fecal material in the colon. Rectal bleeding was assessed based on the presence of diarrhea containing visible blood and on the presence of gross rectal bleeding. DAI values were calculated as{(weight loss score) + (diarrhea score) + (rectal bleeding score)}/n. The DAI was determined by three investigators blinded to the protocol. The clinical parameters used in the present study were chosen to represent the subjective clinical symptoms observed in human UC.
Table 1. Criteria for disease activity index (DAI).
Determination of myeloperoxidase activity
Neutrophil infiltration into the colon was assessed indirectly by measuring myeloperoxidase (MPO) activity. Mid-colon segments were homogenized in EDTA/NaCl buffer (pH 4.7) and centrifuged at 10,000 rpm for 15 min at 4 °C. The pellet was resuspended in 0.5% hexaedcyltrimethyl ammonium bromide buffer (pH 5.4) and frozen in liquid nitrogen and thawed repeatedly three times. Samples were then centrifuged again (10,000 rpm, 15 min, 4 °C) and 25 ul of the supernatant was used for the MPO assay. The enzymatic reaction was assessed by the addition of 25 ul of 1.6 mM tetramethylbenzidine (TMB) in 80 mM, NaPO4, and 100 ul of 0.3 mM H2O2. MPO activity was measured at 560 nm with spectrophotometer and the results are expressed as optical density per milligram of tissue.
Histological processing
Mice were sacrificed at the end of the experiment. The entire colon was dissected and flushed with ice-cold PBS. The resected large intestine was fixed immediately in 4% neutral formalin (Sigma-Aldrich) for 24 h at room temperature and embedded in paraffin to provide sections for histological evaluation. Sections were stained with Hematoxylin and Eosin (H&E; Muto Pere Chemicals, Tokyo, Japan, and Sigma-Aldrich) and viewed under the light microscope. Severity of colitis was evaluated in sections stained with hematoxylin and eosin by two observers blinded to the experimental conditions according to Table .
Table 2. Criteria for assessment of microscopic rectal damage.
Western blot analysis
Segments of colon were washed with phosphate buffered saline (PBS). Distal colons were homogenized in lysis buffer (iNtRON Biotech, Republic of Korea) for 20 min at 4 °C and centrifuged at 13,000 rpm for 5 min. The supernatants were transferred to fresh tubes and protein concentrations were determined using Bio-rad protein assay reagent according to the manufacturer’s instruction. Lysates (50 µg protein) were separated by 10% SDS–PAGE and transferred to membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA), which were blocked with 5% skim milk in PBS-Tween-20 (PBST) for 1 h at room temperature. Membranes were incubated overnight with primary antibodies against phospho-extracellular signal regulated kinase (pERK), phospho-3-Jun, N-terminal kinase (p-JNK), p38, ki67, and β-actin then washed three times with PBST. Blots were incubated with secondary antibody for 1 h at room temperature. Antibody-specific proteins were visualized using an enhanced chemiluminescence detection system (Amersham, Newark, NJ, USA). Protein densities were quantified by densitometry.
Cell culture
RAW 264.7 murine macrophage cell line was obtained from Korean cell line bank (KCLB, Seoul, Korea). RAW 264.7 cells were maintained in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL streptomycin, at 37 °C in a humidified incubator containing 5% CO2. For determination of cell viability, nitric oxide concentration, and inflammatory cytokines (TNF-alpha and IL-6) secretion, the cells were plated at 2 × 105 cell/well in 24-well plates and treated with various concentrations of ES and in the presence of 1 μg/mL LPS for 24 h. For western blotting analysis, the cells were pre-incubated with various concentrations of ES for 1 h and treated with 1 μg/mL LPS for 24 h. ES was dissolved in phosphate buffered saline (PBS, pH 7.4). Cells were treated with PBS as vehicle control.
Cell viability
RAW 264.7 cells were treated with various concentrations of ES (4, 20, 100 μg/mL) for 24 h. Then 5 mg/mL of Thiazolyl blue tetrazolium bromide (MTT) was added for 4 h. The supernatant was removed and the formazan crystal was solubilized in 500 μL DMSO. The absorbance was measured at 570 nm in a multifunctional microplate reader.
Nitrite assay
RAW 264.7 cells were treated with various concentrations of ES (4, 20, 100 μg/mL), after 1 h treated with 1 μg/mL of LPS. 18 h later, the supernatant was collected for nitric oxide (NO) determination. The nitrite accumulated in culture medium was measured as an indicator of NO production based on diazotization reaction using Griess reagent system (SIGMA Aldrich, MO, USA). Nitrite concentration was determined by a standard curve prepared with sodium nitrite dissolved in RPMI 1640 medium without phenol red supplemented with 2% FBS. The absorbance was measured at 570 nm in a multifunctional microplate reader.
Enzyme linked immunosorbent assay
RAW 264.7 cells were cultured at 2 × 105 cell/well in 24-well plates. The cells were pre-treated with various concentrations of ES (4, 20, 100 μg/mL) in the presence of 1 μg/mL of LPS. 24 h after, TNF-alpha levels in the supernatant were measured by ELISA according to the commercial instruction (BD Bioscience, San Diego, CA, USA). A capture antibody, diluted in PBS (1:250 dilution), was incubated overnight 4 °C in 96-well plate. The plate was washed three times with washing buffer (0.05% tween 20 in PBS) and blocked with assay diluent (10% FBS in PBS) for 1 h at room temperature. And then 100 μL of cultured supernatants were added to the wells and incubated at room temperature. After 2 h, the plates were washed three times and incubated for 1 h with a detection antibody solution (1:250 dilution). The plate was washed three times and TMB substrate solution to each well. After 30 min incubation at room temperature in dark place, a stop solution was added to the plate. The optical density of 450 nm was read. The concentrations of the cytokines were calculated using the cytokines’ standard calibration curve.
Statistical analysis
The results are shown as a summary of data from at least three experiments. All results are presented as the mean ± S.E.M. Results were analyzed using Graph Pad Prism version 5.0 program (Graph Pad Software, Inc, La Jolla, CA, USA). One-way analysis of variance with Tukey post hoc test was used to determine statistically significant differences. p < 0.05 was considered significant.
Results
Effects of ES on clinical signs in DSS-induced acute colitis
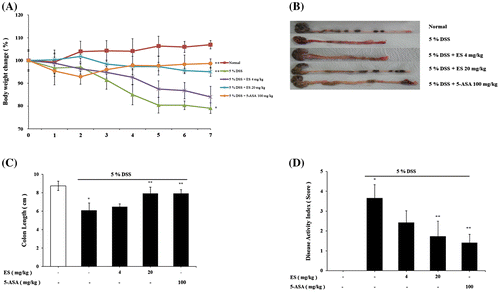
The therapeutic efficacy of ES on experimental colitis was assessed by body weight change, colon length, and disease activity index (DAI). Clinical signs, which were characterized by obvious hyperemia, edema, stool consistency, and ulceration, were observed from the 7th day after DSS administration. To determine whether ES has beneficial effects in DSS-induced acute colitis, author checked body weight, and colon length. Body weight was increased in normal group, whereas dramatically decreased in DSS-treatment group (Fig. (A)). And colon length was shortened in DSS-treatment group compared with normal group as a result of the acute colitis (Fig. (B) and (C)). ES and 5-ASA alleviated the DSS effects on body weight loss. The significant shortening of colon length was observed in DSS-treatment group (6.08 ± 0.81 cm) compared with normal group (7.93 ± 0.68). ES effectively regulated colon length shortening dose dependently (4 mg/kg, 20 mg/kg; 6.47 ± 0.33, 7.92 ± 0.68 cm) compared with DSS-treatment group. ES also attenuated the DSS-mediated increase in DAI scores (Fig. (D)).
Fig. 1. Effect of ES on clinical signs in DSS-induced acute colitis. (A) Body weight was measured at the same time on the experimental days; (B) Representative colons of each group; (C) Average colon length in cm measured after 7 days at the time of sacrificed; (D) Disease activity index score in the five study groups. Values represent mean ± S.E.M. (n = 6). Data were analyzed by Tukey post hoc test (*p < 0.05 versus Normal and **p < 0.05 versus DSS alone).
Effect of ES on MPO activity in DSS-induced acute colitis
The MPO activity in DSS-treatment group was higher significantly than normal group. Increase of MPO activity was detected in DSS-induced colitis in shortened colon, which is indicator of colitis.Citation27) DSS-treatment group showed extensive ulceration, with severe inflammatory cell infiltration. ES and 5-ASA administration significantly decrease MPO levels compared with DSS-treatment group (Fig. (A)).
Fig. 2. Effect of ES on MPO activity and histological changes in DSS-induced acute colitis.
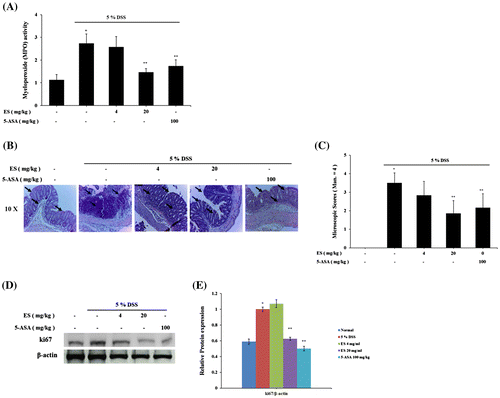
Effect of ES on histological changes in DSS-induced acute colitis
Histological changes in the colons on DSS-induced colitis mice showed mucosal inflammation in the rectum reducing proximal severity. Pathological examinations of colons and rectums were carried out after H & E staining (Fig. (B)). Tissue sections of large intestine showed decrease in crypt epithelium distortion and in mucosal infiltrations, induced by DSS administration, by ES administration. The arrows indicate crypt collapse by DSS administration and recovery by ES administration in colon sections. We found increase of ki67 protein expression on DSS-treatment group at day 7 after DSS administration. The expression of ki67 was significantly reduced in ES treatment group dose dependently (Fig. (D) and (E)).
Effect of ES on COX-2 and iNOS expression in DSS-induced acute colitis
The effects of ES on COX-2 and inducible nitric oxide synthase (iNOS) expression in DSS-induced colitis were determined by western blotting analysis. We found increase of COX-2 and iNOS expression on DSS-treatment group at day 7 after DSS administration. The expressions of COX-2 and iNOS were significantly reduced in ES treatment group dose dependently (Fig. (A)).
Fig. 3. Effect of ES on COX-2, iNOS expression, and NF-κB signal pathway in DSS-induced acute colitis. COX-2 and iNOS levels were determined by western blot analysis. (A) Western blot analysis was used to determine COX-2 and iNOS levels in colonic tissues. Data shown are representative of three independent experiments; (B) Relative protein expression ratios were determined by densitometry; (C) NF-κB, p-IκB-α, and IκB-α levels were determined by western blot analysis; (D) Relative protein expression ratios were determined by densitometry. Values represent mean ± S.E.M. (n = 6). Data were analyzed by Tukey post hoc test (*p < 0.05 versus Normal and **p < 0.05 versus DSS alone).
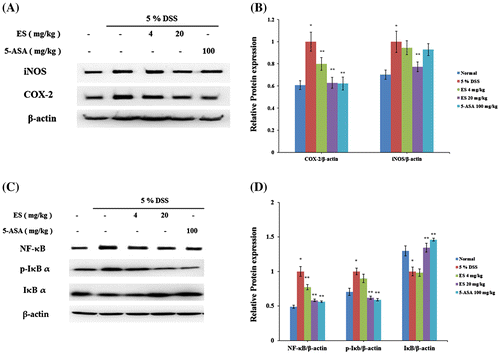
Effect of ES on NF-κB signal pathway
To determine whether ES has remarkable effects to remedy DSS-induced acute colitis, we investigated the expression levels of transcription factors such as NF-κB, IκB-alpha, and IKK-β using western blotting analysis. A significant increase of NF-κB p65 and p-IκBα expressions were observed in DSS-treatment group. In contrast, increase of NF-κB p65 and p-IκBα expressions was suppressed by ES administration (Fig. (B)). These results indicated that ES markedly blocked NF-κB signaling pathway in DSS-induced colitis mice model.
Effect of ES on TNF-α secretion in LPS-stimulated RAW 264.7 cells
The cytotoxicity of ES was measured by MTT assay. Cell viability was not significantly altered by various concentrations (4–100 μg/mL) of ES treatment (Fig. (A)). These results suggested that ES didn’t have cytotoxicity in RAW 264.7 cells. Effect of ES on TNF-α secretion was measured by ELISA. The TNF-α secretion was increased by LPS stimulation compared with vehicle treatment group (vehicle, LPS; 42.52 ± 8.88, 4533.45 ± 56.00 pg/mL). ES reduced LPS-stimulated TNF-α secretion in 20 and 100 μg/mL (20, 100 μg/mL; 3203.02 ± 30.64, 2268.02 ± 41.37 pg/mL) (Fig. (B)). These results suggested that ES had beneficial effect on inflammatory diseases.
Fig. 4. Effect of ES on inflammatory mediators in LPS-stimulated RAW 264.7 cells. (A) RAW 264.7 cells (2 × 105 cells/well) were incubated with ES (4–100 μg/mL) for 24 h. Cell viability was evaluated by MTT colorimetric assay. (B) RAW 264.7 cells (2 × 105 cells/well) were pre-treated with ES (4–100 μg/mL) for 4 h and then stimulated with LPS for 24 h. (C) RAW 264.7 cells (2 × 105 cells/well) were pre-treated with ES (4–100 μg/mL) for 1 h and then stimulated with 1 μg/mL of LPS for 18 h. NO production was evaluated by Griess assay. Values represent mean ± S.E.M. Data were analyzed by Tukey post hoc test (*p < 0.05 versus control and **p < 0.05 versus LPS alone).
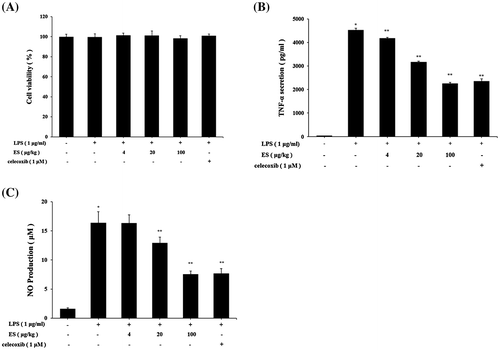
Effect of ES on NO production in LPS-stimulated RAW 264.7 cells
Concentrations of nitrite accumulation in RAW 264.7 cells culture medium were determined by Griess reagent. After macrophages were pre-treated with various concentrations of ES (4, 20, 100 μg/mL) for 4 h, 1 μg/mL of LPS was given for 48 h. NO concentrations were inhibited by ES treatment dose dependently (Fig. (C)).
Discussion
Inflammatory bowel disease (IBD) including Crohn’s disease and ulcerative colitis (UC) is a chronic relapsing intestinal inflammatory disorder.Citation28) The etiology of IBD is still unclear, it is believed that altered immunological functions, genetic susceptibility, and environmental factors can contribute to induce mucosal inflammation on gastrointestinal tract.Citation29) The immune modulators, such as glucocorticosteroids and sulfasalazine, have been widely used for remedy for UC.Citation30) There are various adverse effects to use these therapies, such as vomiting, anemia, and generalized edema. Thus, the use of traditional herbal medicines has been increased for inflammatory chronic diseases.Citation31)
There are over the 20 animal models of colitis. DSS-induced colitis is well-characterized experimental colitis model, drinking DSS for 7–10 days. Using DSS, symptoms of colitis are well-demonstrated with clinical signs, such as body weight loss, stool blood, stool consistency.Citation32) In this study, author focused on treatment strategies, which effectively attenuate the inflammatory reactions without side effects. We investigated the effects of ES on DSS-induced acute colitis in vivo model. We presently demonstrated that ES alleviates clinical signs of acute colitis (weight loss, colon shortening, diarrhea, and occult/gross bleeding) (Fig. ).
In the inflammation reaction, COX-2 production dramatically increases, leading to the increased production of prostaglandins (PGs).Citation33) 5-ASA is used to treat IBD as regulator of COX-2 activation.Citation34) Regulation of COX-2 expression is key point in UC condition. Presently, ES inhibited the DSS-induced activation of COX-2 (Fig. ). The result suggests that anti-inflammatory effect of ES is attributable to the regulation of COX-2 expression in DSS-induced colitis. In addition, we found that ES reduced epithelial injury in colon tissue (Fig. ).
Inflammatory cytokines and chemokines are controlled by the activation of transcriptional foctors. The activation of nuclear factor-kappa B (NF-κB) is important in the pathogenesis of IBD.Citation35,36) IκB phosphorylation and phosphorylation of NF-κB in colon tissues were suppressed by ES oral administration (Fig. ). These results suggested that ES can inhibit the activation of transcription factors in IBD.
In IBD condition, macrophage effectors, neutrophils, and T-cells are activated and release inflammatory cytokines.Citation37) We investigated the anti-inflammatory effect of ES in RAW 264.7 cells. NO was recognized as a mediator and regulator in inflammatory responses.Citation38) In inflammatory progress, a series of cytokines and mediators contributed to evoke inflammation. TNF-α was the important cytokine. Also, inhibition of TNF-α secretion was regarded as a strategy to treat inflammatory disease.Citation39) In this study, ES had outstanding inhibitory effect on LPS-induced NO production dose dependently. Also, TNF-α secretion was decreased by ES treatment dose dependently.
These results suggested that ES has potent anti-inflammatory effects on DSS-induced acute colitis model and inhibitory effects on inflammatory mediator production in macrophages. Furthermore, ES was found as having outstanding therapeutic effects in IBD condition more than 5-ASA. In summary, ES may inhibit inflammatory intestinal disease by regulating the inflammatory mediators such as COX-2 and NF-κB pathway. ES might be a useful therapeutic agent for the treatment of inflammatory disease.
Author Contributions
Yong-Deok Jeon and Ji-Yun Cha performed the in vivo mouse model experiment and analyzed the data. Ji-Yun Cha, Mingjie Xin, Do-Kuk Kim, Hoon-Yeon Lee, and Bo-Ram Kim provided technical and material support. Yong-Deok Jeon, Sung-Woo Hwang, Dae-Ki Kim, and Young-Mi Lee collected the data, undertook the statistical analyses, and wrote the manuscript. Jong-Sik Jin and Young-Mi Lee designed and supervised the study, including editing of the manuscript. All authors contributed to and have approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author.
Funding
This study was supported by grants from Wonkwang University in 2017.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Andoh A, Shioya M, Nishida A, et al. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J. Immunology. 2009;183(1):687–695.10.4049/jimmunol.0804169
- Sands BE. Inflammatory bowel disease : past, present, and future. J. Gastroenterol. 2007;42(1):16–25.10.1007/s00535-006-1995-7
- Mizoguchi A, Mizoguchi E. Inflammatory bowel disease, past, present and future : lessons from animal models. J. Gastroenterol. 2008;43(1):1–17.10.1007/s00535-007-2111-3
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm bowel dis. 2006; 12(supple):S3–S910.1097/01.MIB.0000195385.19268.68
- Rutgeerts P. Protagonist: Crohn’s disease recurrence can be prevented after ileal resection. Gut. 2002;51(2):152–153.10.1136/gut.51.2.152
- Nagura H, Ohtani H, Sasano H, et al. The immuno-inflammatory mechanism for tissue injury in inflammatory bowel disease and Helicobacter pylori-infected chronic active gastritis. Roles of the mucosal immune system. Digestion. 2001;63(1):12–21.10.1159/000051905
- Ludwiczek O, Vannier E, Borggraefe I, et al. Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease. Clin Exp Immunol. 2004;138(2):323–329.10.1111/cei.2004.138.issue-2
- Podolsky DK. Inflammatory bowel disease. N. engl. J. Med. 1991;325(14):417–429.
- Danese S, Semeraro S, Marini M, et al. Adhesion molecules in inflammatory bowel disease: therapeutic implications for gut inflammation. Dig Liver Dis. 2005;37(11):811–818.10.1016/j.dld.2005.03.013
- Kumar GK, Dhamotharan R, Kulkarni NM, et al. Embelin ameliorates dextran sodium sulfate-induced colitis in mice. Int Immunopharmacol. 2011;11(6):724–731.10.1016/j.intimp.2011.01.022
- Xu C, Meng SY, Pan BR. Drug therapy for ulcerative colitis. World J gastroenterol. 2004;10(16):2311–2317.10.3748/wjg.v10.i16.2311
- Matsuoka K, Mizuno S, Hayashi A, et al. Fecal microbiota transplantation for gastrointestinal diseases. Keio J Med. 2014;63(4):69–74.10.2302/kjm.2014-0006-RE
- Chen WX, Ren LH, Shi RH. Enteric microbiota leads to new therapeutic strategies for ulcerative colitis. World J Gastroenterol. 2014;20(42):15657–15663.10.3748/wjg.v20.i42.15657
- Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071.10.1038/ajg.2014.133
- Langhorst J, Wulfert H, Lauche R, et al. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J Crohns Colitis. 2015;9(1):86–106.10.1093/ecco-jcc/jju007
- Ng SC, Lam YT, Tsoi KK, et al. Systematic review: the efficacy of herbal therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38(8):854–863.10.1111/apt.2013.38.issue-8
- Wan P, Chen H, Guo Y, et al. Advances in treatment of ulcerative colitis with herbs: from bench to bedside. World J Gastroenterol. 2014;20(39):14099–14104.10.3748/wjg.v20.i39.14099
- Wang TT, Zhou GH, Kho JH, et al. Vasorelaxant action of an ethylacetate fraction of Euphorbia humifusa involves NO-cGMP pathway and potassium channels. J Ethnopharmacol. 2013;148(2):655–663.10.1016/j.jep.2013.05.025
- Ogata H, Hibi T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr Pharm Des. 2003;9(14):1107–1113.10.2174/1381612033455035
- Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:589–598.
- Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106(2):533–539.10.1016/0016-5085(94)90614-9
- Malizia G, Calabrese A, Cottone M, et al. Expression of leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterology. 1991;100(1):150–159.10.1016/0016-5085(91)90595-C
- Simmonds NJ, Allen RE, Stevens TRJ, et al. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992;103(1):186–196.10.1016/0016-5085(92)91112-H
- Rachmilewitz D, Stamler JS, Bachwich D, et al. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Gut. 1995;36(5):718–723.10.1136/gut.36.5.718
- Kang OH, Kim DK, Cai XF, et al. Attenuation of experimental murine colitis by acanthoic acid from Acanthopanax koreanum. Arch Pharm Res. 2010;33(1):87–93.10.1007/s12272-010-2230-x
- Murthy SN, Cooper HS, Shim H, et al. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporine. Dig Dis Sci. 1993;38(9):1722–1734.10.1007/BF01303184
- Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702.10.1016/0016-5085(90)90290-H
- Dharmani P, Chadee K. Biologic therapies against inflammatory bowel disease: a dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Curr Mol Pharmacol. 2008;1(3):195–212.10.2174/1874467210801030195
- Wu LH, Xu ZL, Dong D, et al. Protective effect of anthocyanins extract from blueberry on TNBS-induced IBD model of mice. Evid Based Complement Alternat Med. 2011;2011:525462.
- Ishiguro Y, Ohkawara T, Sakuraba H, et al. Macrophage migration inhibitory factor has a proinflammatory activity via the p38 pathway in glucocorticoid-resistant ulcerative colitis. Clin Immunol. 2006;120(3):335–341.10.1016/j.clim.2006.05.010
- Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122(6):1592–1608.10.1053/gast.2002.33426
- Rufo PA, Bousvaros A. Current therapy of inflammatory bowel disease in children. Paediatr Drugs. 2006;8(5):279–302.10.2165/00148581-200608050-00002
- Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68–69:165–175.10.1016/S0090-6980(02)00029-1
- Lauritsen K, Laursen LS, Kjeldsen J, et al. Effects of mesalazine on the formation of lipoxygenase and cyclooxygenase products. Adv Exp Med Biol. 1995;371B:1301–1306.
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-κB. Cell Death Differ. 2006;13(5):759–772.10.1038/sj.cdd.4401838
- Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18(18):2195–2224.10.1101/gad.1228704
- Kovacs-Nolan J, Zhang H, Ibuki M, et al. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim Biophys Acta. 2012;1820(11):1753–1763.10.1016/j.bbagen.2012.07.007
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501.10.1016/S0092-8674(01)00237-9
- Rose-John S, Waetzig GH, Scheller J, et al. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11(5):613–624.10.1517/14728222.11.5.613
