Abstract
The regulating role of phosphatidylinositide 3-kinase (PI3K) in benzyl isothiocyanate (BITC)-induced Nrf2 activation, contributing to the inducible expression of cytoprotective genes, was investigated. BITC significantly enhanced the accumulation of Nrf2 as well as autophagic molecules in human colorectal cancer HCT-116 cells. Experiments using a PI3K-specific inhibitor suggested that PI3K plays the key role in the non-canonical Nrf2 activation by BITC.
The nuclear factor-erythroid 2 (NF-E2)-related factor 2 (Nrf2)-kelch-like ECH-associated protein 1 (Keap1)-antioxidant response element (ARE) pathway plays a pivotal role in the inducible expression of cytoprotective genes in response to oxidative stress, environmental xenobiotics, and toxic chemicals.Citation1) The Keap1-Cul3 E3 ubiquitin ligase complex mediates the proteasomal degradation of Nrf2 under basal conditions, whereas, under stress conditions, Nrf2 is translocated into the nucleus in a Keap1-dependent or -independent manner, followed by transcriptional activation of the ARE genes. Oxidative stress and electrophilic chemicals specifically target the reactive cysteine residues of Keap1 and lead to release of Nrf2 from the Nrf2–Keap1–Cul3 complex through its conformational change and thus, enhanced the Nrf2 nuclear translocation (Keap1-dependent canonical pathway).Citation2)
Autophagy is a physiological pathway for lysosomal degradation and recirculation of cellular components, misfolded proteins, and damaged organelles.Citation3) Autophagy is also induced by a variety of stresses, including oxidative stress, ER stress, pathogens, or nutrient deprivation. Selective substrate adaptor proteins, such as p62/sequestosome 1 (SQSTM1), have been shown to facilitate degradation of specific proteins through autophagy.Citation4) p62 directly binds to microtubule-associated protein 1 light chain 3 (LC3), a representative marker of the autophagosome, that is cleaved (LC3-I) and conjugated to phosphatidylethanolamine (LC3-II).Citation5) p62 interacts with ubiquitylated protein aggregates and delivers them to the autophagosomes.Citation6) Although the Keap1-dependent canonical Nrf2 regulation is regarded as the primary mode of action for cytoprotection, p62 also regulates activation of the Nrf2 signaling pathway by Keap1 binding and transferring into the autophagosomes for degradation in an autophagy-dependent manner (non-canonical pathway).Citation7)
Isothiocyanates (ITCs), derived from various cruciferous vegetables, are regarded as potential preventive agents against carcinogenesis, because they are capable of up-regulating the xenotiotic-detoxifying enzymes, inducing apoptosis, and inhibiting cell cycle progression.Citation1) Benzyl isothiocyanate (BITC), an aromatic ITC compound, has been shown not only to inhibit cell proliferation in colorectal cancer cells,Citation8, 9) but to also induce the phase 2 drug-metabolizing enzyme.Citation10) BITC has recently been reported to induce autophagy in human cancer cells.Citation11) Although Keap1 is thought to be the major target for Nrf2 activation by ITCs, such as sulforaphane, the regulating role of autophagy in the BITC-induced Nrf2 activation remains unclear.
In this study, we investigated whether BITC enhanced autophagic molecules, including LC3BII and p62, concomitantly with the Keap1/Nrf2 modulation in human colorectal cancer HCT-116 cells. We also clarified the mediating role of phosphatidylinositide 3-kinase (PI3K) between the autophagy induction and Nrf2 activation by BITC.
Human colorectal cancer HCT-116 cells, obtained from the American Type Culture Collection (Manassas, VA, USA), were maintained in DMEM (Dulbecco’s modified Eagle’s medium, high glucose). All the media were supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin. Cells were grown at 37 °C in an atmosphere of 95% O2 and 5% CO2. BITC was purchased from LKT Laboratories, Inc. (St. Paul, MN, USA). Antibodies against LC3B, LAMP1, and Nrf2 were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Antibodies against Keap1, P62, actin, and the secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Wortmannin and protease inhibitor cocktail were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan) or Nakalai Tesque, Inc. (Kyoto, Japan). Western blotting and RT-PCR experiments were performed previously reported.Citation8) Primers used in the PCR amplification were as follows: human hemeoxygenase 1 (hHO-1), (F) 5’-AAGATTGCCCAGAAAGCCCTGGAC-3’ and (R) 5’-AACTGTCGCCACCAGAAAGCTGAG-3’; human β-actin, (F) 5’-GTCACCCACACTGTGCCCATCTA-3’ and (R) 5’-GCAATGCCAGGGTACATGGTGGT-3’. Densitometric analysis of the bands was carried out using the Image J Software Program (National Institutes of Health, Bethesda, MD, USA). The data are expressed as the means ± standard deviation (SD) of at least three independent experiments and were analyzed using Student`s t-test or Tukey test for comparison between groups. P values of <0.05 were considered to be statistically significant.
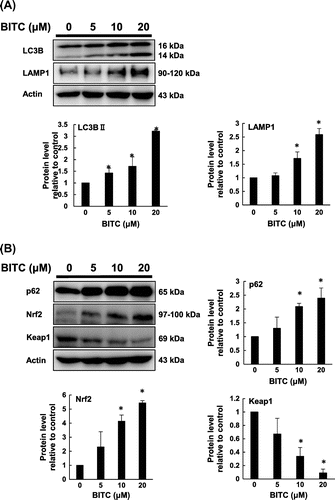
Initial experiments using human colorectal cancer cells showed that BITC enhanced the protein levels of the representative molecules of autophagy, including p62, LC3BII, and LAMP1 in a time-dependent manner up to 12 h (Fig. S1). As shown in Fig. (A) and (B), BITC dose-dependently enhanced the protein levels of LC3BII and LAMP1, concomitantly with the p62 and Nrf2 up-regulation and Keap1 down-regulation. The LC3BII accumulation was actually enhanced by BITC even in the presence of the lysosomal activity inhibitor, bafilomycin A1 (Fig. S2). These results suggested that BITC induced not only autophagy, but also the Nrf2 pathway activation in HCT-116 cells. Immunocytochemistry experiments showed that the incubation of BITC for 12 h resulted in a change in the puncta of LC3B and LAMP1 (Fig. S3(A)) as well as in the MDC stained vacuoles (Fig. S3(B)), further supporting the hypothesis that BITC induces the formation of autophagosome and lysosome.
Fig. 1. Modulating effects on BITC on autophagic (A) and cytoprotective (B) molecules.
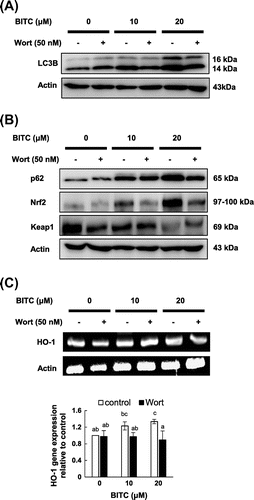
Class III PI3K (PIK3C3/Vps34) has been strongly implicated in autophagic processes in mammals.Citation12) Both LC3B and p62 are prerequisites for the biosynthesis of autophagosome, which is also regulated by PI3K.Citation13) Wortmannin, a selective PI3K inhibitor, has been reported to possess effects on the inhibition of autophagy in human cancer cells.Citation14) As shown in Fig. (A), wortmannin significantly attenuated the BITC-induced accumulation of LC3BII (quantitative data are shown in Fig. S4). Thus, we next examined whether the PI3K inhibitor could affect the Keap1/Nrf2 pathway. As shown in Fig. (B), wortmannin completely impaired the BITC-induced accumulation of the p62 and Nrf2 proteins, coinciding with the full recovery of the down-regulated Keap1. Furthermore, wortmannin significantly impaired the BITC-induced up-regulation of the gene expression of HO-1, one of the representative Nrf2-regulated genes (Fig. (C)). These results suggested that PI3K plays the key role in the non-canonical Nrf2 activation by BITC.
Fig. 2. Effects of wortmannin on the BITC-induced autophagic (A) and cytoprotective (B and C) molecules.
In conclusion, the present data strongly suggested that PI3K plays a pivotal role in association between the Nrf2/Keap1 pathway and autophagy induction in HCT-116 cells. The increasing metabolism and efflux of the drug as well as PI3K mediates the resistance to the chemotherapy drugs.Citation15) The PI3K-mediated pathway is frequently activated and influences survival and drug resistance in a variety of human cancer cells.Citation16) Nrf2 activation can also enhance the resistance of cancer cells to chemotherapeutic drugs.Citation1,17) Therefore, the present results provide evidence that the combination with the PI3K inhibitor is a potential strategy to overcome resistance against food-derived anticancer compounds activating the Nrf2 pathway.
Author contributions
X. L., performed the experiments. N. A.-K., Y. L., B. Z., S. M., T. N., and Y. M. assisted with the experiments and contributed to the discussions. X. L. and Y. N. wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was partly supported by MEXT KAKENHI [grant number 25292073], [grant number16K14928], [grant number17H03818] (YN).
Supplementary materials
The supplemental material for this paper is available at https://doi.org/10.1080/09168451.2017.1374830.
Supplementary-data-YN3.pptx
Download MS Power Point (2.7 MB)supplementary-YN5.docx
Download MS Word (16.7 KB)References
- Nakamura Y, Miyoshi N. Electrophiles in foods: the current status of isothiocyanates and their chemical biology. Biosci Biotechnol Biochem. 2010;74:242–255.10.1271/bbb.90731
- Qin S, Hou D-X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol Nutr Food Res. 2016;60:1731–1755.10.1002/mnfr.v60.8
- Sui X, Jin L, Huang X, et al. p53 signaling and autophagy in cancer: a revolutionary strategy could be developed for cancer treatment. Autophagy. 2011;7:565–571.10.4161/auto.7.6.14073
- Ichimura Y, Kominami E, Tanaka K, et al. Selective turnover of p62/A170/SQSTM1 by autophagy. Autophagy. 2008;4:1063–1066.10.4161/auto.6826
- Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145.10.1074/jbc.M702824200
- Jiang T, Harder B, Rojo de la Vega M, et al. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88:199–204.10.1016/j.freeradbiomed.2015.06.014
- Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426.10.1146/annurev-pharmtox-011112-140320
- Abe N, Hou DX, Munemasa S, et al. Nuclear factor-kappaB sensitizes to benzyl isothiocyanate-induced antiproliferation in p53-deficient colorectal cancer cells. Cell Death Dis. 2014;5:e1534.10.1038/cddis.2014.495
- Sakai R, Yokobe S, Abe N, et al. Luteolin overcomes resistance to benzyl isothiocyanate-induced apoptosis in human colorectal cancer HCT-116 cells. J Food Drug Anal. 2012;20:389–393.
- Nakamura Y, Ohigashi H, Masuda S, et al. Redox regulation of glutathione S-transferase induction by benzyl isothiocyanate: correlation of enzyme induction with the formation of reactive oxygen intermediates. Cancer Res. 2000;60:219–225.
- Xiao D, Bommareddy A, Kim SH, et al. Benzyl isothiocyanate causes FoxO1-mediated autophagic death in human breast cancer cells. PLoS ONE. 2011;7:e32597.
- Itakura E, Kishi C, Inoue K, et al. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–5372.10.1091/mbc.E08-01-0080
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405.
- Zhou XY, Luo Y, Zhu YM, et al. Inhibition of autophagy blocks cathepsins-tBid-mitochondrial apoptotic signaling pathway via stabilization of lysosomal membrane in ischemic astrocytes. Cell Death Dis. 2017;8:e2618.10.1038/cddis.2017.34
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58.
- Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta. 2015;1855:104–121.
- Huang Y, Li W, Su ZY, et al. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem. 2015;26:1401–1413.10.1016/j.jnutbio.2015.08.001
