Abstract
Persimmon is a very delicious fruit and the leaves of this tree are used as a traditional drug. This study aimed to investigate the effects of drying method (hot air and freeze-drying), extraction temperature (80, 90 and 100 °C) and extraction time (10, 30, 60 and 120 min) and harvest stage (flowering and fruiting) on the antioxidant contents and antioxidant activity of persimmon leaves. The results showed that the highest antioxidants were obtained in both methods of drying. Also, 100 °C for 120 min of extraction gave the highest antioxidant contents, but with no significant difference compared to 90 °C for 60 min of extraction. Persimmon leaves collected during flowering stage had the maximum amount of antioxidants compared to the fruiting stage. Finally, it can be said that persimmon leaves harvested during flowering stage and treated by hot air drying with these extraction conditions (90 °C for 60 min) are richer in bioactive compounds.
Graphical abstract
Harvest time, drying method and extraction condition had a significant effect on the antioxidants properties of persimmon leaf.
Persimmon (Diospyros kaki) is widely distributed in Eastern Asian countries, particularly China, Japan, and Korea. The fruit of persimmon is eaten fresh or dry, whereas the leaf is well known to be used in pharmaceuticals, cosmetics as well as beverages. Due to functional properties, the leaf of persimmon has been used in the treatment of paralysis, stroke, frostbite, hemorrhage, constipation, burns, and apoplexy.Citation1)
Antioxidants are broadly used in the food production because they help to reduce the rate of oxidation. Bioactive compounds are isolated either directly or indirectly from different natural sources and the role of those compounds for improving health by reducing the risk of many diseases. Persimmon leaf has attracted much attention due to their high antioxidant contents such as phenols, tannins, flavonoids, natural acids, ascorbic acid, caffeine, oligomers, and chlorophyll.Citation2,3) The polyphenols in persimmon leaf are widely investigated due to their antiallergic, anti-inflammatory, antibacterial, and skin-lightening properties. Moreover, it has beneficial effects on diabetes.Citation4) However, flavonoids (kaempferol, catechin, and quercetin) show an extensive range of therapeutic activities, such as anticancer, anti-inflammatory, and antiallergic properties.Citation5,6) Also, the flavonoid is one of the major components in Naoxinqing (NXQ) tablet and it is an approved traditional Chinese medicine made from the extracts of persimmon leaf to cure coronary diseases.Citation1) On the other hand, persimmon leaf is richer in tannin content and it has beneficial effects on human body particularly, lowering blood pressure.Citation7) Besides, persimmon leaf has shown robust radical-scavenging activity which can be attributed to the presence of epicatechin, catechin, chlorogenic acid, epigallocatechin, gallic acid, and caffeic acid.Citation8) Hence, persimmon leaf may be used as a functional ingredient in food matrix because of the high antioxidant contents. Already it has been incorporated into different food matrix as a natural food additive such as candies, rice cake, cookies, athlete’s foot socks and soaps, sushi ingredient,Citation9) and so on.
Drying techniques are able to preserve the sensory quality and antioxidants. This is because it helps to reduce the moisture content of fresh samples that might alter the chemical, physical, and microbiological characteristics. Also, it helps to increase the shelf-life of the products and minimizes the costs of packaging, transportation as well as storage. Freeze-drying and hot air drying are the very common drying treatments used in food preservation. Freeze-drying method considered the most superior type of drying to preserve nutritional value as well as color. It has been stated that freeze-drying technique increases the extraction of antioxidants from various goods compared to hot air drying,Citation10) though it has always been considered as the most expensive technique. Hence, it is important to classify the optimal drying conditions for preparation of dried persimmon leaves for further processing steps.
There is a rising attention in formulating effective and naturally friendly extraction procedures. The extraction procedure is greatly influenced by the temperature, time of extraction, types of solvent, pH, particle size, and solid-to-liquid ratio.Citation11) Organic solvents are widely used to extract antioxidants from the plant on a large-scale. It has been considered that organic solvents are not naturally friendly, due to the difficulty in getting rid of all trace solvent from the extracts. Water is a non-toxic, non-flammable, and readily available solvent, where safe extraction can be exercised.
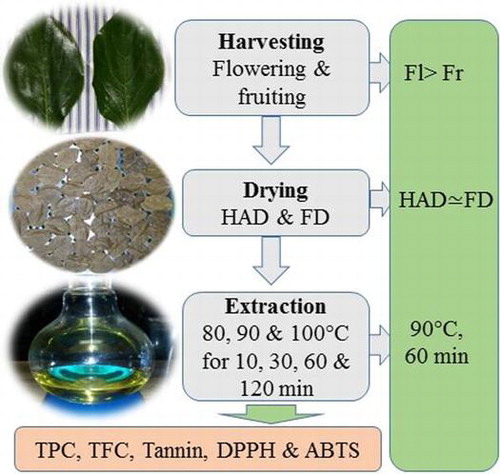
This study aimed to determine the effect of drying methods (freeze-drying and hot air drying at 100 °C for 30 min), extraction conditions (80, 90, and 100 °C for 10, 30, 60, and 120 min), and harvest time (flowering and fruiting) on the antioxidant properties of persimmon leaves. These antioxidants could be used in the functional food as well as pharmaceutical industries.
Materials and methods
Materials
The mature persimmon leaves were taken from the persimmon experiment station, Sangju, South Korea on May 25 (flowering time) and June 25 (fruiting time) in 2016. The 35 trees were tagged to collect leaf samples and the cultivar used was “Sangju-dungsi.”
The following chemicals were used for analysis: Folin–Ciocalteu reagent, gallic acid, and catechin were obtained from Sigma Chemicals (St Lous, MO, USA). 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (DPPH) and 2, 2-diphenyl-1-picrylhydrazyl (ABTS) were purchased from TCI Co., Ltd. (Tokyo, Japan). Sodium nitrite, sodium carbonate, and aluminum chloride were purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan). Methanol, ethanol, and sodium hydroxide were obtained from Duksan Pure Chemicals Co. Ltd. (Ansan, Korea). Vanillin was purchased from Samchun Chemical Co., Ltd. (Seoul, Korea). All other chemicals were analytical grade.
Aqueous extraction from persimmon leaves
The samples were blanched at 100 °C for 2 min and then dried using hot air dryer at 100 °C for 30 min and freeze-dryer (industrial vacuum freeze-dryer) for 72 h. The dried persimmon leaves were ground and sieved to obtain the particle size ≤1 mm. The extraction was performed with a product to water ratio of 1:10 (w/w) at 80, 90, and 100 °C for 10, 30, 60, and 120 min. The extracts were filtered using Whatman filter paper (4 Whatman™; 150 mm∅). Filtrates were collected and kept at 4 °C until required.
Color measurement
The color was assessed using a color difference meter (UltraScan Pro Spectrophotometer, HunterLab Inc., Reston, Virginia, USA). To standardize the device black and white tiles were used as a standard. Dried persimmon leaves powder was transferred to a measurement cell and measured L*-, a*-, and b*-value through the computerized system.
Determination of total phenolic content
The total phenolic content (TPC) was measured as described by Singleton and RossiCitation12) with slight modifications. Briefly, 0.2 mL of the extracts was added to 1 mL of 10% Folin–Ciocalteu’s phenol reagent in the flask and left for 10 min, then mixed with 0.8 mL of 7.5% sodium carbonate (Na2CO3). The solution was left at room temperature for 2 h, then its absorbance was measured at 765 nm using a spectrophotometer. Gallic acid with the concentration range from 0 to 200 mg/L was used for the standard curve and results were expressed as mg gallic acid equivalents per gram of dry matter (mg GAE/g d.m.).
Determination of total flavonoid content
The content of flavonoid was determined based on the method described by Dewanto et al.Citation13) Concisely, 0.25 mL of the sample was added to 1 mL of distilled water in a test tube, then mixed with 75 μL of a 5% sodium nitrite (NaNO2) and the solution was kept at room temperature for 6 min. Then, 150 μL of a 10% aluminum chloride (AlCl3) was mixed and kept for a further 5 min, followed by the addition of 0.5 mL of 1 M sodium hydroxide (NaOH) and 2 mL of distilled water. The absorbance was measured instantly at 510 nm using a spectrophotometer. The results were expressed as mg of (+)—catechin equivalent (mg CE/g d.m.) per gram of dry matter.
Determination of tannin content
The tannin content was measured as described by Xu and Chang.Citation14) Concisely, 0.5 mL of the sample, 3 mL of a 4% methanol vanillin solution, and 1.5 mL of hydrochloric acid (conc.) were mixed. The solution was kept at room temperature for 15 min and the absorbance was measured at 500 nm using a spectrophotometer against methanol as a blank. The results were expressed as mg catechin equivalents (mg of CE/g d.m.) per gram of dry matter using the calibration curve of (+)—catechin.
DPPH radical-scavenging activity
DPPH radical-scavenging assay was assessed using the method described by Shahidi et al.Citation15) with some modifications. To, 0.1 mL of the sample diluted in methanol (1 mL extract/5 mL methanol) was mixed with 3.9 mL of a methanolic mixture of DPPH (methanol: water; 80:20) (0.025 mg/mL). The solution was shaken and left in the dark at ambient temperature for 30 min. The absorbance was measured at 510 nm using a spectrophotometer. The antioxidant activity (%) of the samples was calculated according to the following formula:
where, Acontrol is the absorbance of DPPH solution without the sample.
ABTS radical-scavenging activity
The ABTS radical-scavenging activity was measured as described by Thaipong et al.Citation16) with some modifications. Briefly, 7.4 mM ABTS (0.203 g/50 mL) solution and 2.6 mM (0.035 g/50 mL) potassium persulfate solution were mixed in equal quantities and left under darkness at room temperature for 12 h. Then the ABTS stock solution was diluted by adding 1 mL ABTS mixture with 24 mL ethanol to obtain an absorbance at 734 nm of 1.5 ± 0.02 using a spectrophotometer. The fresh ABTS mixture was set up for each assay. Lastly, 0.3 mL sample was added with 2.7 mL of the ABTS mixture and its absorbance was measured after 7 min at 734 nm. The scavenging activity was expressed as a percentage (%) according to the following formula:
Statistical analysis
The data were reported as the mean ± standard error of triplicate measurements. Analysis of variance (ANOVA) was accomplished to estimate the effect of process variables (drying method, extraction condition, and harvest time). A significant difference (p < 0.05) within means was analyzed using the Statistical Analysis System (SAS 9.3) program.
Results and discussion
Color
The color values of persimmon leaf powder are shown in Table . The L* (lightness) and b* (yellowness) of freeze-dried leaf were the highest, whereas the opposite scenario was seen in hot air-dried leaf powder (p < 0.05). Generally, the factors affecting the color during drying are the high-temperature and long drying time. However, a loss of redness (a* value) was found in freeze-dried samples whereas the opposite was true in hot air-dried leaf (p < 0.05). On the other hand, the color values remained almost the same in both harvest times (flowering and fruiting).
Table 1. Effect of drying and harvest time on the color characteristics of persimmon leaves.
Effect of drying technique on the extraction of antioxidant compounds
The selected drying treatments used in this experiment were hot air drying (HAD) and freeze-drying (FD). Results were obtained from the samples harvested on May 25 (flowering time). The result from the study demonstrated that there were no significant differences between HAD and FD samples with respect to bioactive compounds (Tables and ). For example, the total phenolic compound showed no significant difference between HAD and FD. Generally, FD considered the ideal technique for preserving phenolic contents, but in this experiment, it was showing the similar tendency as hot air drying. Though, Harbourne et al.Citation17) reported that FD of willow resulted in lower levels of phenolic contents than thermal drying methods. This could be described by the fact that drying time was longer during FD (72 h) compared to HAD (30 min), might have caused degradation of some polyphenol. Also, during FD enzymatic activity increase upon thawing might have affected degradation of some polyphenol.Citation18) Moreover, lyophilization process possibly enriches the browning reaction which is responsible for the oxidation of phenolic compounds by the activity of the enzyme. On the other hand, the higher temperature in HAD destructs the tissue and allowed more extraction of polyphenol. This is because; drying has an ability to break the cellular components of foods to release the polyphenol.
Table 2. Effect of extraction time and temperature on the antioxidant contents and antioxidant activity of the extracts obtained by hot air drying.
Table 3. Effect of extraction time and temperature on the antioxidant contents and antioxidant activity of the extracts obtained by freeze-drying.
In the case of total flavonoids, the similar tendency was found in dry leaf extracts, where FD samples showed the similar level of flavonoids as shown in HAD samples. These results are in agreement with the findings reported by Alonzo-macías et al.Citation19) on dried strawberry, who stated that FD samples revealed the similar flavonoids as HAD samples. The loss of flavonoids could be due to the polymerization and oxidation during the FD treatment. However, the effect of different drying techniques on tannin content was similar to total phenolic as well as flavonoids. HAD yielded the same tannin content as shown in the FD. Stewart et al.Citation20) recommended that the FD should be avoided in tannin research, where air drying can be used for tannin identification. This is because, during FD treatment, there might be a possibility of a decrease in the content of tannin because of degradation of few compounds.
On the other hand, the percentage inhibition of DPPH and ABTS radical-scavenging activities was similar in both HAD and FD. The loss of antioxidant activities could be due to the enzymatic degradation by the Maillard reaction during FD treatment. Previous studies have reported that the FD and HAD samples showed the similar trends with respect to antioxidants activity from dried strawberryCitation19) and raspberry powders.Citation21) However, the results demonstrate that the percentage (%) inhibition of DPPH was lower than those of ABTS radical-scavenging activity at the same condition. Lee et al.Citation22) stated that ABTS has a higher scavenging activity when compared with DPPH activity from the natural plants extracts.
A good correlation was found between the concentration of total phenol versus antioxidants activity and flavonoids versus antioxidants activity in both methods of drying (Table ). Results obtained from the Tables and indicate that phenols and flavonoids were found to have less correlation with ABTS activity compared to DPPH activity. Therefore, the application of drying was able to increase the extraction of antioxidant from persimmon leaf because the conditions of drying significantly affect the final product. According to the results (Tables and ) obtained in this study, it could be said that the effective drying method for optimum antioxidant extraction capacity from persimmon leaf is by hot air, though there were no differences between HAD and FD. This is because FD is considered an expensive method yet similar results can be obtained from HAD within a short time and less energy consumption.
Table 4. Correlation coefficients between antioxidant activity vs. total phenol and total flavonoid.
Effect of temperature and time on the extraction of antioxidant compounds
The selected temperatures for extraction were 80, 90, and 100 °C. Results were obtained from the samples collected on 25th May (flowering stage). The results showed that extraction temperature had a significant effect (p < 0.05) on the persimmon leaf extracts (Tables and ). The highest phenolic and tannin contents were achieved at extraction temperatures of 90 and 100 °C. This could be due to the more open structure of the dry leaf, which allows easy penetration of solvent at high-temperature, thus, positively affecting the extraction process. Corrales et al.Citation23) reported that heat application can break the structure of the phenolic-matrix and affect the membrane chemical structure of plant tissues. However, increased extraction temperature may help to release more inactive compounds and increase solvent losses from the extracts. In the present study, total flavonoid content raised when the extraction temperature increased to 90 °C, and subsequently declined when the infusion temperature reached 100 °C (p < 0.05). However, it should be noted that rising infusion temperature above certain levels may help possible degradation of flavonoids. This is because the high-temperature may help to solvent loss and lead to the degradation of the flavonoid content. A previous study reported that flavonoids degrade in water at the temperature of 100 °C or more.Citation24) On the other hand, when the temperature increased from 80 to 90 °C, the extraction efficiency of DPPH and ABTS scavenging activity increased significantly (p < 0.05) and the highest level of antioxidant activity were found at 90 and 100 °C. Abdul Rahim et al.Citation25) found that antioxidant activity of Plectranthus amboinicus extracts increased until 100 °C of extraction and then declined to a lower level at 120 °C.
Extraction time (10, 30, 60, and 120 min) had a significant effect (p < 0.05) on total extraction yield (Tables and ). A time increment from 10 to 120 min resulted in a 1.5-fold increase in bioactive compounds. The results showed that the antioxidant contents increased gradually with the increase of extraction time till 60 min and then it remained constant between 60 and 120 min of extraction. This phenomenon ensured that the equilibrium of extraction was achieved. It could be described by the Fick’s second law of diffusion where the concentrations of the solute are in equilibrium after a certain time. Our results are in accordance with Silva et al.Citation26) who stated that shorter time (1 h) shows the same amount of phenolic content as long time (5 h). Moreover, Naczk and ShahidiCitation27) reported that prolonged infusion time may allow extending the exposure of bioactive compounds to oxygen and light, which led to degradation of phenolic contents. However, the highest DPPH and ABTS scavenging activity were observed at 1 h and began to decrease at 2 h (p < 0.05) of extraction. Our findings are in harmony with Azman Abdul Rahim et al.Citation25) who found that 60 and 120 min were the best time periods for extracting the antioxidant content from Plectranthus amboinicus extracts. Also, prolonged infusion time led to degradation of bioactive compounds, which caused in lower scavenging activity.
Taking into account these facts, an extraction temperature of 90 °C and extraction time of 60 min were selected as the best extraction conditions for extracting antioxidants from persimmon leaf. Based on our result, it could be said that 90 °C for 60 min was the optimum extraction condition, while similar results were found at the high-temperature for a long time. This is because high-temperature and the long time may increase solvent loss through vaporization and enhance the extraction method cost from the industrial perspective.
Effect of harvest time on antioxidants content of persimmon leaves
In order to detect the effectiveness of antioxidants content from persimmon leaves at different harvesting time, a control experiment was conducted. Results were obtained at the optimal extraction condition (90 °C for 60 min) by hot air drying treatment (Table ). Our results showed that persimmon leaves collected on May 25 (flowering stage) contained the highest amount of antioxidants and antioxidant activities than those harvested on 25th June (fruiting stage) (p < 0.05). The amount of phenolic content, flavonoid content, tannin content, DPPH, and ABTS radical-scavenging activity was 32.88, 19.14, 64.06, 21.02, and 20.43%, respectively, lower in late June compared to late May. For example, 90.41 ± 0.74 and 60.68 ± 0.52 mg GAE/g dm of phenolic content and 30.67 ± 1.33 and 24.80 ± 0.73 mg CE/g dm of flavonoid content were obtained during flowering and fruiting stage, accordingly. This result agrees with the results of Jung and JeongCitation28) and Chung et al.Citation29); they reported that persimmon leaf collected in May had the higher level of polyphenol and flavonoids among different harvesting stages. However, no clarification was provided about that fluctuation of antioxidant compounds in persimmon leaves. The possible causes of the differences among harvest periods can be associated with plant physiological characteristics, environmental conditions mainly temperature, light intensity, and the various phases of fruit growth and development. Peixoto et al.Citation30) reported that the amount of phenolic may alter according to the growth phase, the part of the plant, and the environment condition. Moreover, the amount of tannin was 47.00 ± 0.77 and 16.89 ± 0.44 mg CE/g dm during flowering and fruiting stages, respectively. Chung et al.Citation29) described that the persimmon leaf harvested in early June contained the highest level of tannin among different harvesting times. Probably this might be due to differences in soil types, fertilizers, climate, and management. Also, Scogings et al.Citation31) reported that the content of phenolics and tannins were greater in all species in the early stage. On the other hand, the percentage of DPPH was 48.86 ± 0.96 and 38.59 ± 0.28 and the percentage of ABTS was 88.17 ± 1.11 and 70.16 ± 0.66 during flowering and fruiting stages, accordingly. This variation could be a result of different morphological, ecological, genetic, and environmental aspects. Zou et al.Citation32) reported that early stage of mulberry leaves had the highest phenolics and antioxidant activity compared to the late stage.
Table 5. Effect of harvest time on antioxidants content in persimmon leaves.
WangCitation33) reported that pre-harvest condition particularly temperature, climate, light intensity, types of soil, fertilization, compost, the amount of CO2 in the atmosphere, all can affect the antioxidant contents of the harvested fruits. However, the potential reason of these fluctuations of antioxidant contents and antioxidant activities in persimmon leaves are still ambiguous. Consequently, it can be said that the probable cause for this transformation could be the movement of antioxidants from leaves to fruits during fruiting.
Conclusions
The results of this study reveal that hot air drying can be explored as an optimum method for treating persimmon leaves retaining the maximum amount of antioxidants due to the short time of drying and less energy consumption. Although, no significant difference was observed between hot air and freeze-drying methods regarding antioxidant contents. This is because the operating cost of freeze-drying is too high which is not suitable for the industrial point of view. The optimum infusion conditions for the extraction of bioactive compounds of the persimmon leaves were projected and validated at 90 °C for 60 min. Our results suggest that flowering stage is the best harvest time, with respect to antioxidant contents from persimmon leaves. These antioxidants could be used in the nutraceutical and pharmaceutical industries. More work is required to study the potential differences of antioxidants level in persimmon leaves from different harvest stages.
Author contributions
All authors discussed the results and implications and commented on the manuscript at all stages.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors are grateful to persimmon experiment station (Sangju) for supplying persimmon leaves during the study.
References
- Xie C, Xie Z, Xu X, et al. Persimmon (Diospyros kaki L.) leaves: a review on traditional uses, phytochemistry and pharmacological properties. J Ethnopharmacol. [Internet]. 2015;163:229–240. Available from: https://doi.org/10.1016/j.jep.2015.01.007
- Matsuo T, Ito S. The chemical structure of kaki-tannin from immature fruit of the persimmon (Diospyros kaki). Agric Biol Chem. 1978;42:1637–1643.
- Jo C, Son JH, Shin MG, et al. Irradiation effects on color and functional properties of persimmon (Diospyros kaki L. folium) leaf extract and licorice (Glycyrrhiza Uralensis Fischer) root extract during storage. Radiat Phys Chem. 2003;67:143–148.10.1016/S0969-806X(02)00443-7
- Wang L, Xu ML, Rasmussen SK, et al. Vomifoliol 9-O-α-arabinofuranosyl (1 → 6)-β-D-glucopyranoside from the leaves of Diospyros Kaki stimulates the glucose uptake in HepG2 and 3T3-L1 cells. Carbohydr Res. [Internet]. 2011;346:1212–1216. Available from: https://doi.org/10.1016/j.carres.2011.04.021
- Gao Y, Wang Y, Ma Y, et al. Formulation optimization and in situ absorption in rat intestinal tract of quercetin-loaded microemulsion. Colloids Surf B Biointerfaces. 2009;71:306–314.10.1016/j.colsurfb.2009.03.005
- Rogerio AP, Dora CL, Andrade EL, et al. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol Res. 2010;61:288–297.10.1016/j.phrs.2009.10.005
- Funayama S, Hikino H. Hypotensive principles of Diospyros kaki leaves. Chem Pharm Bull. 1979;27:2865–2868.10.1248/cpb.27.2865
- Chen G, Xue J, Xu S-X, et al. Chemical constituents of the leaves of Diospyros kaki and their cytotoxic effects. J Asian Nat Prod Res. [Internet]. 2009;9:347–353. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17613620
- Ladas SD, Kamberoglou D, Karamanolis G, et al. Systematic review: Coca-Cola can effectively dissolve gastric phytobezoars as a first-line treatment. Aliment Pharmacol Ther. 2013;37:169–173.10.1111/apt.2012.37.issue-2
- Pinela J, Barros L, Dueñas M, et al. Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: effects of drying and oral preparation methods. Food Chem. 2012;135:1028–1035.10.1016/j.foodchem.2012.05.038
- Durling NE, Catchpole OJ, Grey JB, et al. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007;101:1417–1424.10.1016/j.foodchem.2006.03.050
- Singleton V, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158.
- Dewanto V, Wu X, Adom KK, et al. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014.10.1021/jf0115589
- Xu BJ, Chang SKC. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007;72:S159–66.
- Shahidi F, Liyana-Pathirana CM, Wall DS. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006;99:478–483.10.1016/j.foodchem.2005.08.009
- Thaipong K, Boonprakob U, Crosby K, et al. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–675.10.1016/j.jfca.2006.01.003
- Harbourne N, Marete E, Jacquier JC, et al. Effect of drying methods on the phenolic constituents of meadowsweet (Filipendula ulmaria) and willow (Salix alba). LWT - Food Sci Technol. [Internet]. 2009;42:1468–1473. Available from: https://doi.org/10.1016/j.lwt.2009.05.00510.1016/j.lwt.2009.05.005
- Ashie INA, Simpson BK. Application of high hydrostatic pressure to control enzyme related fresh seafood texture deterioration. Food Res Int. [Internet]. 1996;29:569–575. Available from: https://www.sciencedirect.com/science/article/pii/S096399699600012910.1016/S0963-9969(96)00012-9
- Alonzo-Macías M, Cardador-Martínez A, Mounir S, et al. Comparative study of the effects of drying methods on antioxidant activity of dried strawberry (Fragaria Var. Camarosa). J Food Res. 2013;2:92–107.10.5539/jfr.v2n2p92
- Stewart JL, Mould F, Mueller-Harvey I. The effect of drying treatment on the fodder quality and tannin content of two provenances of Calliandra calothyrsus Meissner. J Sci Food Agric. 2000;80:1461–1468.10.1002/(ISSN)1097-0010
- Si X, Chen Q, Bi J, et al. Comparison of different drying methods on the physical properties, bioactive compounds and antioxidant activity of raspberry powders. J Sci Food Agric. 2016;96:2055–2062.10.1002/jsfa.2016.96.issue-6
- Lee BW, Lee JH, Gal SW, et al. Selective ABTS radical-scavenging activity of prenylated flavonoids from Cudrania tricuspidata. Biosci Biotechnol Biochem. 2006;70:427–432.10.1271/bbb.70.427
- Corrales M, Toepfl S, Butz P, et al. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innov Food Sci Emerg Technol. 2008;9:85–91.10.1016/j.ifset.2007.06.002
- Srinivas K, King JW, Howard LR, et al. Binary diffusion coefficients of phenolic compounds in subcritical water using a chromatographic peak broadening technique. Fluid Phase Equilib. [Internet]. 2011;301:234–243. Available from: https://doi.org/10.1016/j.fluid.2010.12.00310.1016/j.fluid.2010.12.003
- Azman Abdul Rahim MS, Salihon J, Yusoff MM, et al. Effect of temperature and time to the antioxidant activity in Plecranthus amboinicus lour. Am J Appl Sci. 2010;7:1195–1199.10.3844/ajassp.2010.1195.1199
- Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55:381–387.10.1016/j.seppur.2007.01.008
- Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542.10.1016/j.jpba.2006.04.002
- Jung W, Jeong J. Change of antioxidative activity at different harvest time and improvement of atopic dermatitis effects for persimmon leaf extract. Korea J Herbol. 2012;27:41–49. Korean.10.6116/kjh.2012.27.1.41
- Chung SH, Moon KD, Kim JK, et al. Changes of chemical components in persimmon leaves during growth for processing persimmon leaves tea. Korean J Food Sci Technol. 1994;26:141–146. Korean.
- Peixoto Sobrinho TJDS, Silva CHTPD, Nascimento JED, et al. Validation of spectrophotometric methodology for quantify flavonoid content in Bauhinia cheilantha (Bongard) Steudel. Rev Bras Ciências Farm. [Internet]. 2008;44:683–689. Available from: https://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-93322008000400015&lng=pt&nrm=iso&tlng=pt Portuguese.10.1590/S1516-93322008000400015
- Scogings PF, Dziba LE, Gordon IJ. Leaf chemistry of woody plants in relation to season, canopy retention and goat browsing in a semiarid subtropical savanna. Austral Ecol. 2004;29:278–286.10.1111/aec.2004.29.issue-3
- Zou Y, Liao S, Shen W, et al. Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in Southern China. Int. J Mol Sci. 2012;13:16544–16553.10.3390/ijms131216544
- Wang SY. Effect of pre-harvest conditions on antioxidant capacity in fruits. Acta Hortic. 2006;712 I:299–306.10.17660/ActaHortic.2006.712.33
