Abstract
Since the discovery of its role in the CO2 fixation reaction in photosynthesis, RuBisCO has been one of the most extensively researched enzymes in the fields of biochemistry, molecular biology, and molecular genetics as well as conventional plant physiology, agricultural chemistry, and crop science. In addition, the RuBisCO and RuBisCO-like genes of more than 2000 organisms have been sequenced during the past 20 years. During the course of those studies, the origin of the RuBisCO gene began to be discussed. Recent studies have reported that the RuBisCO gene emerged in methanogenic bacteria long before photosynthetic organisms appeared. The origin of similar early genes might have allowed this gene to overcome changes in global environments during ancient and recent eras and to participate in the fixation of 200 GT of CO2 annually. In this review, I focus on several points that have not been discussed at length in the literature thus far.
Graphical abstract
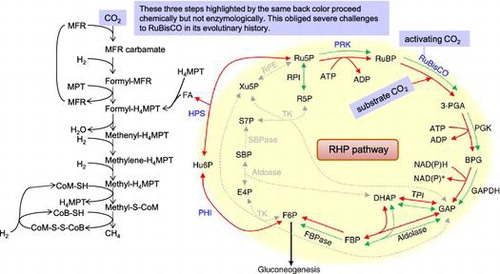
Proposed reductive hexulose-phosphate pathway and related metabolic processes in Archaea.
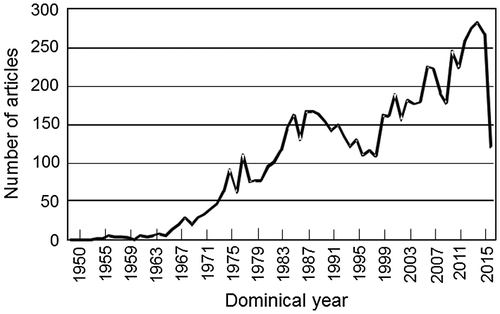
The CO2 fixation reaction in photosynthesis was first described in a paper published in 1950 (Fig. ).Citation1) Afterward, general enzymatic chemistry studies were conducted, but the enzymatic properties revealed therein did not explain the gas exchange of individual plant leaves.Citation2) Since 1970, the oxygenase reaction of ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCO) and its activation by CO2 in the CO2 fixation reaction have been clarified.Citation3–5) Based on these important discoveries, a biochemical model of plant photosynthesis gas exchange was constructed in 1980,Citation6) and the photosynthetic CO2 fixation reaction advanced to become an integrated science discussed in fields such as biochemistry, plant physiology, crop science and ecology. In addition, RuBisCO has been studied in molecular biology for the past two decades and has been studied extensively as a field intimately related to food production and environmental management. In particular, during the past 10 years, the number of papers on RuBisCO published annually has increased, exceeding 250 papers in 2014 (Fig. ).
Fig. 1. The number of RuBisCO-related articles published in each year after the discovery of the photosynthetic CO2 fixation reaction in 1950.
Since the whole picture of photosynthetic carbon metabolism was elucidated in 1970, researchers around the world have attempted to remedy important and unavoidable problems to enhance plant productivity. Given this opportunity to summarize my thinking on how to increase the CO2 fixation ability of RuBisCO, I would like to suggest a future direction to which my research strategies have yet to venture.
I. On measures to enhance plant productivity
There are 26,000 to 35,000 genes in plant genomes, and the greatest productivity of plants is obtained when they function maximally by successfully detecting surrounding information. With this production level taken as the maximum production potential, the yield of plants under field conditions reaches only approximately 20–25% of their production potential because they are subjected to various kinds of environmental stresses.Citation7) Naturally, it is important to know the types of environmental stress to which plants are subjected and the response mechanisms of these plants to those types of stress.
In general, living organisms, including plants, do not always employ the best genes in their evolutionary processes. After differentiation to form unique molecular lines, organisms cannot exchange genes with species of other lineages due to reproductive isolation in mating. As described below with respect to RuBisCO, the genomic sequence of many organisms is being decoded by advances in genome science, and comprehensive consideration with physiological and biochemical knowledgeCitation8) demonstrates that, in this reproductive isolation, plant photosynthesis has adapted to the long-term changes in the global environment from a carbonated atmosphere to an oxygenated one. Given this historical background, I believe there are two strategies to enhancing photosynthetic function. One involves directly strengthening the functions of rate-limiting metabolic steps in the photosynthetic carbon reduction cycle and increasing the catalytic activity of those steps to improve productivity potential.Citation9) The other strategy, which involves improving the intracellular environment so that catalytic potentials of overall rate-determining enzymes can be maximized, has been little discussed.
II. Molecular basis of plant function enhancement
II.i. CO2 fixation
The fact that photosynthesis is uniquely restricted by the inconvenient properties of the RuBisCO enzyme is still widely accepted globally.Citation9) In 1970, it was found that RuBisCO also catalyzes the oxygenase reaction in addition to the carboxylase reaction of ribulose bisphosphate (RuBP).Citation3,10) In plants, this reaction combined with the CO2-releasing phenomenon in photorespiration causes photosynthesis efficiency to decrease by 30–50%.Citation11) In addition, the reaction rate of RuBisCO is approximately 0.1–1% of that of ordinary enzymes, and its low affinity to substrate CO2 also causes plant photosynthesis to progress only at a slow rate. Currently, information about the structure–function relationship of RuBisCO in various organisms in nature is important for the successful protein engineering of the enzyme.Citation12)
Photosynthetic RuBisCO is roughly classified into two forms based on amino acid sequence and protein structure.Citation12,13) Form I adopts the L8S8 structure, which is composed of eight large subunits (L) and eight small subunits (S); the green-like form I RuBisCO exists in plants, eukaryote green algae, cyanobacteria and photosynthetic bacteria, and the red-like form I RuBisCO exists in red algae and some bacteria. Form II adopts the L2n structure, which is composed only of L subunits, and exists in photosynthetic bacteria, chemical bacteria and dinoflagellates. The enzymatic properties of RuBisCO vary greatly depending on the evolutionary lineage of the species. The kCcat of the carboxylase reaction of higher plant RuBisCO, which includes the green-like RuBisCOs of form I, ranges from 2.9 to 4.2 catalytic site−1 s−1; for green algae enzymes, this value ranges from 2.3 to 5.4 site−1 s−1, and for cyanobacterial enzymes 2.6 to 13.2 site−1 s−1. However, red-like RuBisCOs have a kCcat ranging from 1.3 to 3.4 site−1 s−1, and in photosynthetic bacteria, red-like RuBisCOs have a value ranging from 2 to 3.2 site−1 s−1. Additionally, the kCcat is 3.5 to 7.3 for the photosynthetic bacterial enzyme, which is form II. In particular, the CO2/O2 specificity factor (or relative specificity, Srel), which is an important characterization among the kinetic parameters of RuBisCO, is (kCcat/KCm)/(kOcat/KOm), where kCcat and kOcat are the maximum carboxylase reaction and oxygenase reaction rates, respectively, and KCm and KOm are the km values for CO2 and O2, respectively.Citation14) RuBisCO with a high Srel has a strong ability to discriminate in favor of CO2 in a reaction system containing both CO2 and O2, meaning that it is specialized in the carboxylase reaction compared with the oxygenase reaction. The Srel of higher plant RuBisCO, which is the green-like form I RuBisCO, ranges from 70 to 94; this range is lower in the green algal form I (54 to 83) and further lower in the cyanobacterial RuBisCO (38 to 56).Citation12–14) Additionally, the Srel of photosynthetic bacteria ranges from 26 to 53. In contrast, the Srel of the red-like RuBisCO of photosynthetic bacteria ranges from 105 to 238; in particular, the RuBisCO of red algae shows higher values than does green-like RuBisCO. RuBisCO from the primitive red alga Galdieria partita shows the highest Srel among RuBisCOs that have been found thus far.Citation15) Note that increasing affinity for CO2 is an important factor in an increase in Srel. However, as a result, the CO2 fixation rate decreases. Since RuBisCO does not have binding sites for the gaseous substrates,Citation13) it has been proposed that the observed higher Srel values are due to the characteristics of the enzyme that bind the carboxylase reaction intermediate to the catalytic site much more tightly than the oxygenase reaction intermediate. Therefore, there is a so-called tradeoff between affinity for CO2 and reaction rate (Fig. ).Citation8) This trade-off is why the Srel value has a positive relationship with the reactivity of the carboxylase reaction but has the opposite relationship with the reactivity of the oxygenase reaction.
Fig. 2. Natural variation in the kinetic parameters of RuBisCO measured in vitro at 25 °C.Citation8)
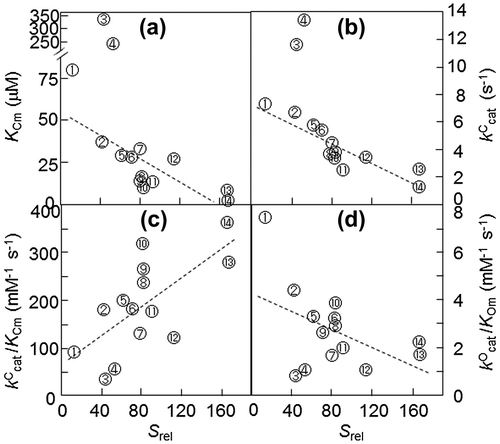
Thus, the fact that the Srel of RuBisCO differs depending on the species is believed to be indicative of the adaptation of the properties of RuBisCO to the environment in which each organism evolved on Earth. Indeed, in photosynthetic organisms such as bacteria that have RuBisCOs showing lower Srel than plant RuBisCO, many grow in anaerobic environments; in aerobic environments, these lower-Srel RuBisCOs are subjected to the inhibition of carboxylase activity by oxygen. Algae attained an ability to induce the CO2 concentrating mechanism (CCM) to increase intracellular CO2 concentrations to inhibit the oxygenase reaction.Citation16) However, in higher plants, it appears that molecular evolution increased the Srel of RuBisCO to fix CO2 even on land where O2 is present in greater amounts than CO2.Citation12) Furthermore, in C4 plants, by evolving a metabolic CCM—the so-called C4 pathway—the CO2 concentration around RuBisCO is elevated.
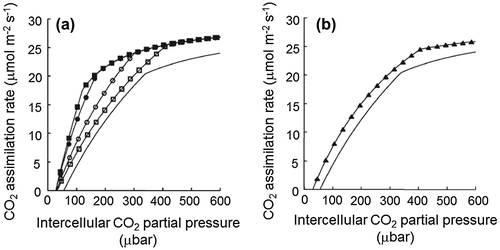
In fact, we can simulate how the Srel of RuBisCO affects plant photosynthesis using the photosynthetic model of Farquhar.Citation17) In this model, there are two phases in the dependency of CO2 fixation rate on the intercellular CO2 concentration. One is the RuBisCO activity-limiting phase under low CO2 concentrations; as the CO2 concentration increases, the dependency shifts to the RuBP regeneration rate-determining phase. Using this model, a simulation of the CO2 fixation rate in plant leaves with high-Srel RuBisCO is shown in Fig. .Citation12) Compared with the wild type, when the Srel of RuBisCO increases by a factor of 2, the CO2 fixation rate is expected to increase at all CO2 concentrations, and the CO2 compensation point decreases from 50 to 30 μbar. In particular, the increase in Srel either by increasing kCcat or by decreasing KCm causes a dramatic increase in the CO2 fixation rate under low CO2 concentrations. As such, in a plant using RuBisCO that has such a high Srel, the CO2 concentration necessary for achieving the same CO2 fixation rate as wild type is lower, and the water use efficiency in photosynthesis is higher. These simulation results suggest that increasing the Srel of RuBisCO is an important point for improving plant photosynthetic CO2 fixation rate.Citation8,12)
Fig. 3. Effect of the properties of RuBisCO on photosynthetic CO2 assimilation rates.Citation12)
The carboxylase reaction of RuBisCO proceeds sequentially as follows. Substrate RuBP binds to the activated RuBisCO reaction center, and substrate CO2 is added to the C2 of enediol-type RuBP, which is generated by the enzymatic withdrawal of the C3 proton of RuBP, after which 3-keto-2-carboxyarabinitol bisphosphate is produced. In the case of the oxygenase reaction, 3-keto-2-peroxyarabinitol bisphosphate is generated.Citation13) Because there are no binding sites for these gaseous substrates in the RuBisCO protein, the addition rate of the gaseous substrates should depend simply on the concentrations of the substrates, and the reported large difference in Srel among RuBisCOs of different origins cannot be explained by the affinity of the enzyme to the gaseous substrates.Citation8,12) These major differences depend on the process by which reaction products are formed after the gaseous substrate binds.
II.ii. Activation of RuBisCO
For all RuBisCO enzymes to show activity, a CO2 molecule binds to the ε-amino group of Lys-201 to form a carbamyl anion. This CO2 molecule is involved only in the activation and does not become the substrate. Mg2+ is further coordinated to the carbamyl anion.Citation4,5,18) Thereafter, an enzymatically active RuBisCO Lys-201-NH-COO–-Mg2+ (ECM) ternary complex is formed, and the complex is stabilized by the carboxylate groups of Asp-203 and Glu-204. Citation19,20) Mg2+ and the activator CO2 play important roles, such as withdrawing H+ from the C3 of the substrate RuBP and stabilizing the carboxylate of the CO2-fixing reaction intermediate.Citation20) To activate RuBisCO in vitro, 20 mM Mg2+ and 0.1 to 0.2 mM CO2 are both necessary, but the stromal CO2 concentration during photosynthesis can be as low as 5 to 7 μM,Citation21) and the reported high RuBisCO activation levels cannot be explained without one or more effectors.
Ogren’s group at the University of Illinois explained this gap eloquently. RuBisCO activase, which belongs to the AAA + family, is involved in the activation of RuBisCO and the removal of endogenous inhibitors that bind strongly to RuBisCO.Citation22,23) Interestingly, in the presence of RuBisCO activase, even in higher plants under saturated light in normal atmosphere, RuBisCO is 80–90% activated. Increasing amounts of RuBisCO by sufficient use of nitrogen fertilizer and overexpression of the S subunit gene of RuBisCO in plant leaves decreases this activation ratio.Citation24,25) In contrast, if the amount of RuBisCO is forcibly reduced by the antisense method, the activation ratio of RuBisCO increases.Citation26) Moreover, increasing the amount of activase in rice decreases the content of RuBisCO and decreases the CO2 fixation activity.Citation27) The mechanism by which such trade-offs involving RuBisCO occur in these cases has not yet been described.
III. Enhancement of photosynthetic function by promoting the activation of RuBisCO
III.i. Details of the activation mechanism
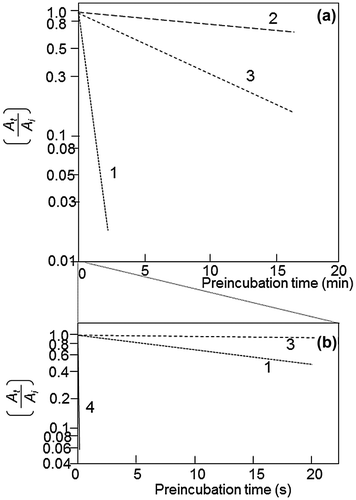
The activation reaction of RuBisCO can be described as follows. The RuBisCO protomer binds activator CO2 on the ε-amino group of Lys-201 to make a carbamate ion. Mg2+ ion binds to the carbamate ion to form the ECM complex, where E is the RuBisCO protomer, C is the activator CO2, and M is Mg2+. The carbamate ion formation is very slow, and the binding of Mg2+ proceeds very quickly.Citation5) When 44 μM RuBisCO protomer was activated with 112 μM CO2 at 10 °C, the activation reached an equilibrium in 10 min in activation experiments with RuBisCO.Citation5) The rate constant of CO2 binding to the amino group of the model compound monoethanolamine (MEA) is 6.1 × 103 M−1 s−1 at 30 °C.Citation28) Calculated using the rate constant for MEA carbamylation, the equilibrium would have been reached in 3.2 s with the concentrations of the RuBisCO protomer and CO2 used by Lorimer et al.Citation5) The primary reaction rate at which CO2 is liberated from MEA carbamate is 29.8 s−1, and its half-life is 0.023 s, while the half-life at which activated RuBisCO is inactivated under inactivation conditions is 20 s (Fig. ). The difference between the carbamylation rate of MEA and the ε-amino group of Lys-201 cannot be explained by the difference in reaction temperatures used by these groups.Citation25,28) Interesting research results related to this discrepancy have been reported by Vater et al.Citation29) In their analysis using p-toluidinonaphthalene sulfonate, the fluorescence emission from the sulfonate reflects the binding of the probe to the hydrophobic regions of RuBisCO. These authors found that an increase in fluorescence emission reflects a conformational change of the RuBisCO protein by the binding of either CO2 or Mg2+ or both to RuBisCO; this conformational change may be important in RuBisCO activation, as discussed later.
Fig. 4. Effector-dependent suppression of the deactivation of RuBisCO.
III.ii. RuBisCO activation and RuBisCO activase
Discoveries in numerous pioneering studies of the structure-function relationship of RuBisCO have clarified the formation of an ECM:Glu-203:Asp-204 triangle contained in the periphery of Lys-201, and the tightly fixed ECM allows RuBisCO to function and produce a CO2 fixation reaction.Citation20) It is also important that RuBisCO activase is deeply involved in this process. Recent reports from the Max Planck Research Institute have posed important challenges to the mechanism of this activation.Citation30,31)
For some time now, it has been known that the concentration of RuBisCO activase in chloroplasts is considerably lower than that of RuBisCO.Citation32) When RuBisCO activase exerts its function on RuBisCO, the activase functions by forming a hexamer.Citation30,31) The RuBisCO holoenzyme (L8S8) content is 3 μmol m−2, and the hexameric activase (A6) content is 0.57 μmol m−2.Citation33) Mate et al.Citation34) determined these values to be 2.5 and 0.15 μmol m−2, respectively. In antisense experiments for the activase, the gas exchange rate was not affected until the activase amount was reduced to 1/5 of the wild-type level;Citation33–35) therefore, the amount of the activase required for 1 μmol m−2 of the RuBisCO holoenzyme ranges from 0.04 to 0.06 μmol activase hexamer m−2 for photosynthesis to proceed at normal speed.
In the in vitro activation of RuBisCO from the ER form, in which the RuBisCO holoenzyme concentration and activase hexamer concentration are almost the same, nearly full activity was attained.Citation36) The reason for the difference in the required concentrations of the activase in vivo and in vitro is an important research target.
III.iii. Activation in the presence of sugar phosphates and reduced nicotinamide adenine dinucleotide phosphate
Several sugar phosphates have an interesting influence on the activation of RuBisCO. Glucose 6-phosphate and ribulose 5-phosphate are considered negative effectors and have no effect on the activation, but 6-phosphogluconate (6PG), fructose 1,5-bisphosphate (FBP), reduced nicotinamide adenine dinucleotide phosphate (NADPH) and sodium phosphate promote the activation of RuBisCO and are considered positive effectors.Citation37–39) The RuBisCO substrate RuBP itself also contributes to the prolonged binding of the activator CO2 to Lys-201.Citation40) In a detailed analysis with 6PG,Citation41) the Ki based on competitive inhibition kinetics against RuBP was 8.5 μM, while the dissociation constant in the equilibrium binding analysis was 37 μM. Plotting of the concentration of 6PG toward the activation level of RuBisCO produced a saturation curve, and the obtained half-saturation concentration for activation was 50 μM (Fig. ).
Fig. 5. Double reciprocal plots between the preincubation concentration of the effectors on the activation of RuBisCO activity.
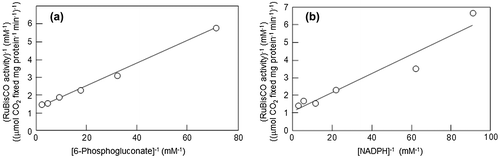
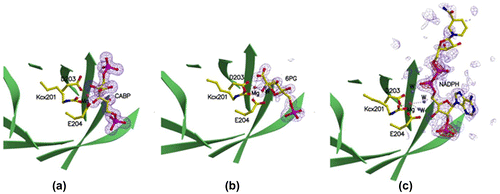
The km of RuBisCO for RuBP is 20 μM.Citation41) This implies that 6PG merely binds to the RuBP binding site competitively with respect to RuBP but adopts a binding mode that differs from that of the competitive inhibition mode in the absence of RuBP. NADPH is likewise a competitive inhibitor against RuBP,Citation41) but there is no difference between the Ki and the half-saturation concentration in the activation of NADPH unlike 6PG. The binding mode of NADPH may be different from that of RuBP, as discussed below. This point was also consistent with the results from the structural analysis of the complexes of rice RuBisCO and its positive effectors. In rice RuBisCO crystallized in the presence of 40 mM 6PG or 40 mM NADPH as well as atmospheric CO2 and 40 mM MgCl2, the active site structures were markedly different from those deduced from the above kinetic analyses.Citation42) Sugar phosphate was bound to the catalytic site, where the ε-amino group of Lys-201 was carbamylated, and further Mg2+ was bound to the carbamate anion. The position of the carbamyl-group oxygen of Lys-201 stabilizing this Mg2+ and the positions of the Asp-203 and Glu-204 carboxyl group oxygens in the 6PG-binding enzyme form closely overlapped with those in the NADPH-binding form. The structures of the rice RuBisCO complexed with 6PG and NADPH are very similar to the catalytic center structure of spinach RuBisCO in the activated state. One small difference between these two enzymes is that the carboxyl groups of 6PG and the oxygen of the hydroxyl group on C2 are directly coordinated to Mg2+ in the former, but in the NADPH-binding enzyme, the adenylate phosphate oxygen and the oxygen of adenylate ribose C3 stabilize Mg2+ via two water molecules (Fig. ). However, in this state, where two phosphate groups of RuBP are more than 9.4 Å apart, RuBisCO displays open-state conformations. In a closed-state conformation, where two phosphate groups are 9.1 Å apart or less, as seen in the quaternary complex between ECM and the transition state analog 2-carboxyarabinitol bisphosphate, the N-loop, 60s loop, loop 6 and C-loop of L are in ordered states. However, in the NADPH-binding form, these loops are in disordered states. In the 6PG-binding form, only the 60s loop is ordered.
Fig. 6. Structures of the catalytic sites of (a) 2-CABP, (b) 6PG-, and (c) NADPH-bound RuBisCOs with electron density maps of the ligands.Citation42)
It is interesting to analyze the functions regarding the activation of 6PG and NADPH from deactivation kinetics (Fig. ). When activated RuBisCO is diluted with buffer devoid of CO2 or with the ethylenediaminetetraacetate (EDTA)-containing buffer, deactivation of RuBisCO proceeds with a long half-time of approximately 20 s.Citation41) This may be because the conformational change induced by the binding of activator CO2 and Mg2+ to RuBisCO stabilizes this activated conformation even in the presence of EDTA. Then, the addition of 6PG or NADPH to this RuBisCO deactivation buffer containing EDTA caused the deactivation half-time to increase from 20 s to 1800 and 300 s, respectively. In other words, these positive effectors maintained RuBisCO in the activated conformation for a longer period, even in the presence of EDTA.
Another point to discuss is that the concentration of NADPH in chloroplasts is much lower than the concentration of the RuBisCO protomer.Citation43) As 6PG is synthesized only at night,Citation44) this will be discussed later in detail.
III.iv. RuBisCO: a conformation-memorizing enzyme?
As discussed above regarding references 5 and 28, the carbamate formation of the ε-amino group of Lys-201 for the activation of RuBisCO requires more than 10 min in the presence of 44 μM RuBisCO protomer, 112 μM CO2, and 20 mM MgCl2, while the chemical reaction of 44 μM MEA and 112 μM CO2 reached the equilibrium in 3.2 s; meanwhile, CO2 liberation from carbamylated MEA proceeded 870 times faster than the decarbamylation reaction of the activated enzyme. Such slow activation/deactivation empirically measured for RuBisCO can be reasonably explained if we assume that the conformational change of the RuBisCO protein is involved in these processes as discussed above. The detailed analysis of RuBisCO activation/deactivation by Badger and LorimerCitation41) showed that when transferred to EDTA-containing buffer, RuBisCO activated in the presence of NADPH was decarbamylated 13,000 times slower than the chemical decarbamylation (Fig. (b)). When RuBisCO activated by NADPH was placed in a reaction medium containing saturated concentrations of RuBP and CO2 in the presence of EDTA, RuBisCO showed full activity without any delay, indicating that the substrate RuBP quickly removed NADPH from the catalytic site, but surprisingly, exogenously added EDTA could not deprive Mg2+ or of course activator CO2 from the ECM complex. In other words, activated RuBisCO must have the capability to maintain or memorize its activated conformation for some period of time. It has been thought that the reported concentration of NADPH (0.25–0.5 mM)Citation43) was too low compared with the concentration of the RuBisCO protomer in chloroplasts (4 mM). FBP and phosphate are reported to be present at approximately 5.4 and 12 mM, respectively, in chloroplastsCitation45,46), and may similarly maintain the activated state of RuBisCO as does NADPH.Citation37–39)
The minimum required amount of RuBisCO activase hexamer required for 1 μmol m−2 of the RuBisCO holoenzyme in chloroplasts ranges from approximately 0.04 to 0.06 μmol m−2 RuBisCO activase, as discussed above. Activase is necessary not only for activating RuBisCO but also for removing various sugar phosphate inhibitors from the RuBisCO active center. It is conceivable that the conformation-memorizing characteristics of the activated state of RuBisCO play an important role to exert this function even with lower concentrations of RuBisCO activase. Considering these points, I propose that the activation state may be maintained for a while in vivo, aided by various positive effectors and by the conformation-memorizing character of activated-form RuBisCO.
III.v. Accelerating photosynthetic functions by optimizing the functional environment of RuBisCO in chloroplasts
To clarify the rate-determining step and the rate-limiting factor of the Calvin cycle that affect photosynthetic ability and carbon partitioning, a number of transformants with suppressed expression of various enzymes have been produced by the antisense method.Citation47) Moderate suppression of glyceraldehyde 3-phosphate dehydrogenase,Citation48) phosphoribulokinase,Citation49) aldolase,Citation50) and fructose 1,6-bisphosphatase (FBPase)Citation51) did not cause photosynthesis to decrease. These results indicate that many enzymes of the Calvin cycle are present in sufficient quantities to maintain photosynthetic capacity at the protein level. On the other hand, suppression of RuBisCO expression is directly linked to a decrease in photosynthetic ability under light saturation, but under unsaturated light conditions, RuBisCO is not the only rate-limiting factor for photosynthetic performance.Citation52,53)
In antisense plants with sedoheptulose 1,7-bisphosphate (SBPase),Citation54) the photosynthetic ability decreases proportionally to a decrease in SBPase activity. In lines in which the SBPase activity decreased to 29% of that of wild type, the photosynthetic activity decreased to 36% of that of wild type. Based on these reports, it is clear that the reduction in RuBP regeneration ability in the Calvin cycle affects carbon assimilation and growth, and in particular, the change in SBPase level has a significant effect on those Calvin cycle processes.
Information from such studies has encouraged attempts to overexpress the genes of these enzymes. Cyanobacteria have a unique Calvin cycle enzyme not present in higher plants.Citation55) Since this enzyme catalyzes the reactions of both FBPase and SBPase with one protein, it was named fructose 1,6-/sedoheptulose 1,7-bisphosphatase (FBP/SBPase). In actuality, when the activity of both of these enzymes in chloroplasts was increased by introducing the cyanobacterial FBP/SBPase gene into tobacco chloroplasts via AgrobacteriumCitation56) or chloroplast DNACitation57) directly, the photosynthetic CO2 fixation activity of individual leaves under a light intensity of 400 μmol photons m−2 s−1 increased by 30 to 50%, and the total biomass of the plant increased by 50 to 80%. Little effect of the introduced FBP/SBPase on the photosynthetic activity was observed at 200 μmol m−2 s−1 or less, and the promotion of photosynthesis was pronounced at 700 μmol m−2 s−1 or more. In transformed plants with introduced FBP/SBPase, the RuBP concentration almost doubled, and the activation ratio of RuBisCO increased by 30%. I believe that this is one of the causes of increased photosynthetic activity in the transformant. The activity of RuBisCO in chloroplasts is controlled by RuBisCO activase as mentioned above. A high concentration of RuBP is necessary for the function of RuBisCO activase,Citation58) and the phenotype of this transformed plant seems to be due to the activation of the RuBP regeneration system by introduction of FBP/SBPase. Much higher concentrations of RuBP also further increase the activity of RuBisCO.Citation59) The Km value of RuBisCO for RuBP is approximately 20 μM in the catalytic reaction, and the maximum activity is obtained between 0.5 and 1 mM. We found that when the RuBP concentration was 2 mM or higher, the activity further increased by 30%. In actuality, in biochemical analyses by equilibrium ligand binding and conformational change with p-toluidinylnaphthalene sulfonate, RuBisCO had one activity-regulating site per protomer that binds various sugar phosphates in addition to the catalytic site.Citation60,61) Also, reported stromal concentrations of RuBP have sometimes exceeded 6 mM.Citation45,62)
A similar photosynthesis-promoting effect has also been observed when the NAD kinase-2 gene was introduced into chloroplasts of rice via Agrobacterium. The introduction of NAD kinase-2 caused the concentration of NADPH to increase to levels 1.5 times higher than those in the non-transformant and the concentration of RuBP to increase 2.7-fold.Citation63) It seems that both of these increase the activation of RuBisCO as discussed in this review, although the authors of this paperCitation63) presented a different way of thinking.
IV. A challenge regarding the area that we have not been able step into thus far: RuBisCO is still plagued by its origin
Even today, when the nucleotide sequences of the genomes of more than 4000 species have been completely decoded, research fields that make good use of these large data remain to be developed. For example, the photosystem II protein D1 was an enzyme that appeared when photosynthetic organisms attained the oxygen-generating function, but the gene located at the root of the phylogenetic tree of the D1 gene has not been found in non-photosynthetic organisms.Citation64) An ancient oxygen-generating photosynthetic organism must have appeared using such a gene.
In the case of the RuBisCO rbcL gene, until approximately 1990, the phylogenetic root of the rbcL gene was considered to be the cyanobacterial rbcL, similarly to the D1 gene, and the tree only determined the position of evolution among photosynthetic organisms. When the existence of archaea was proposed and the genome of archaea began to be deciphered, an authentic archaean rbcL gene, the gene of an enzyme that can fix CO2 on its acceptor RuBP, was found in 1999.Citation65,66) In 2007 the metabolic function of this gene became clear.Citation67) Further decoding of archaebacterial and eubacterial genomes revealed a great number of genes that encode proteins lacking some residues essential for RuBisCO reactions but that were very similar to RuBisCO in other ways. The phylogenetic tree of these rbcL-related genes revealed the occurrence of an enormous and diverse rbcL-related gene family different from forms I to III of the rbcL families. These genes constituted form IV, which was distinct from the form I to III families. The protein of the form IV family was named RuBisCO-like protein (RLP). The functional relationship between forms I to III and form IV became the next intriguing question.Citation68)
Bacillus subtilis RLP (bsRLP), which belongs to the form IV family, has only 11 residues among the 19 catalytic residues of RuBisCO, and its amino acid sequence shares only 18.3% homology with that of a form II RuBisCO of the photosynthetic bacterium Rhodospirillum rubrum. We found that bsRLP catalyzed the enolization reaction between C1 and C2 of 2,3-diketo-5-methylthiopentyl-1-phosphate, which is structurally very similar to RuBP.Citation69) This finding revealed the overall functions of the members of the enormous RuBisCO rbcL gene families that had been clarified in previous genome decoding studies.Citation70) However, the origin of the rbcL gene families, including bsRLP, remains to be clarified.
Tabita et al.Citation71) reported an interesting point by creating an evolutionary phylogenetic tree of rbcL family genes using four software packages for the evolutionary phylogenetic tree generation. The form III rbcL gene consisted of two subfamily forms, III-1 and III-2, but there was a clade consisting of other form III rbcL genes in the vicinity of the III-1 and III-2 groups. The newly found group had the gene for the eukaryotic phosphoribulokinase (PRK).Citation72) In our most recent findings, photosynthetic PRK genes were classified into two groups: one from photosynthetic bacteria and the other from plant-type oxygenic phototrophs, such as plants, algae, and cyanobacteria.Citation73) PRK homologs from methanogenic archaea including Methanospirillum hungatei showed approximately 30% amino acid sequence identity with PRKs in photosynthetic organisms and formed a clade clearly distinct from those of bacterial and plant-type PRKs in a phylogenetic tree. The enzymatic properties of the archaeal PRKs were different from those of photosynthetic bacteria and plant-type oxygenic phototrophs. The archaeal form III consisted of two subfamilies: III-a and III-b. All methanogenic archaea included in the III-a and III-b forms have the RuBisCO gene and compose the primitive Calvin pathway, also known as “the reductive hexulose-phosphate (RHP) pathway.”Citation73) Because this pathway is the main pathway for methane synthesis, formaldehyde (FA), which would be provided from the RHP pathway, is likely to have also played an important role (Fig. ).
Fig. 7. Proposed RHP pathway and related metabolic processes in archaea.
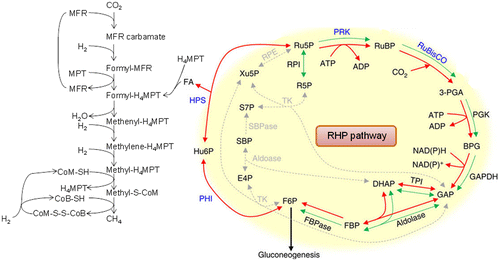
In primeval times, where methane-producing archaea began to synthesize methane, the atmosphere was almost saturated with CO2 and almost free of oxygen.Citation74) Under such circumstances, methanogenic archaea synthesize N-carboxymethanofuran (carbamate) by a chemical reactionCitation74) rather than by an enzymatic reaction of CO2 at a concentration of several tens of millimoles per liter, and methane is formed by a consecutive reduction of N-carboxymethanofuran (carbamate) by hydrogen gas (Fig. ). As described above, the reaction rate constant of MEA and CO2 is 6.1 × 103 M−1 s−1 at 30 °C. Since the CO2 concentration in water in equilibrium with 100% atmospheric CO2 is 23 mM, assuming that the intracellular concentration of methanofuran is 1 μM, the formation rate of N-methanofuran carbamate is 1 μmol s−1.Citation75) RuBisCO, which appeared in the ancient environment that contained high concentrations of CO2 without oxygen, may not have required any catalytic residue for the carbamylation of the ε-amino group of Lys-201 and for the chemical addition of gaseous CO2 on the C2 of the endiolate of RuBP.Citation20) I suppose that all of the extant RuBisCOs perform these two chemical reactions with CO2 during photosynthesis and that this inconvenience is forcing this enzyme and photosynthetic organisms to pay a large cost.
V. Epilogue
Through the subsequent appearance and exuberance of oxygenic photosynthesis, the concentration of oxygen in the atmosphere has reached a high of 210,000 ppm, and the atmospheric CO2 concentration has decreased to 0.04% or less. In this review, I have touched on the history of the bold adaptations of RuBisCO to these changes. As a result, RuBisCO has suffered a tradeoff of a drastic decrease in kCcat but has overcome this adversity via the algae CCM. Higher plants mainly strengthened the promoters of multi-copy rbcS to develop green leaves as a factory to utilize RuBisCO as the CO2 fixation enzyme. Perhaps detailed analyses of the evolutionary process of the gene of this enzyme from the ancient origins of RuBisCO to the present form may provide hints toward improving current photosynthesis. I look forward to these discoveries continuing in the future.
Funding
This work was supported by Japan Science and Technology Agency [grant number CREST]
Disclosure statement
No potential conflict of interest was reported by the author.
Acknowledgements
I would like to dedicate this review to late professors emeritus Shozaburo Kitaoka, Akira Wadano, and Yasushi Kai, who were my distinguished co-researchers during the past thirty years.
Notes
This review was written in response to the author’s receipt of the JSBBA Senior Scientist Award in 2014.
Abbreviations: Bs-RLP, Bacillus subtilis RuBisCO-like protein; CCM, CO2-concentrating mechanism; EDTA, ethylenediaminetetraacetate; FA, formaldehyde; FBP, fructose 1,6-bisphosphate; FBPase, fructose 1,6-bisphosphatase; FBP/SBPase, fructose 1,6-/sedoheptulose 1,7-bisphosphatase; MEA, monoethanolamine; 6PG, 6-phosphogluconate; PRK, phosphoribulokinase; RHP, reductive hexulose-phosphate, RLP, RuBisCO-like protein; RuBP, ribulose 1,5-bisphospahte; Srel, relative specificity; SBPase, sedoheptulose 1,7-bisphosphatase.
References
- Benson AA, Bassham JA, Calvin M, et al. The path of carbon in photosynthesis. V. Paper chromatography and radioautography of the products. J Assoc Chem Soc. 1950; 72(4): 1710–1718.10.1021/ja01160a080
- Paulsen JM, Lane MD. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966;5(7):2350–2357.10.1021/bi00871a025
- Ogren WL, Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol. 1971;230(13):159–160.10.1038/newbio230159a0
- Laign WA, Ogren WL, Hageman RH. Bicarbonate stabilization of ribulose 1,5-diphosphate carboxylase. Biochemistry. 1975;14(10):2269–2275.
- Lorimer GH, Badger MR, Andrews TJ. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976;15(3):529–536.10.1021/bi00648a012
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149(1):78–90.10.1007/BF00386231
- Boyer JS. Plant productivity and environment. Science. 1982;218(4571):443–448.10.1126/science.218.4571.443
- Tcherkez GG1, Farquhar GD, Andrews TJ. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc Natl Acad Sci USA. 2006;103(19):7246–7251.10.1073/pnas.0600605103
- Yokota A, Shigeoka S. Engineering photosynthetic pathways. In: Bohnert HJ, Nguyen HT, editors. Bioengineering and molecular biology of plant pathways, Volume 1. In: Lewis NG, executive editor. Advances in plant biochemistry and molecular biology. Dordrecht: Elsevier; 2008. p. 81–105.10.1016/S1755-0408(07)01004-1
- Bowes G, Ogren WL, Hageman RH. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971;45(3):716–722.10.1016/0006-291X(71)90475-X
- Ku SB, Edwards GE. Oxygen inhibition of photosynthesis: I. temperature dependence and relation to O2/CO2 solubility ratio. Plant Physiol. 1977;59(5):986–990.10.1104/pp.59.5.986
- Ashida H, Yokota A. Increasing photosynthesis/RuBisCO and CO2-concentrating mechanisms. In: Murray M-Y, editor. Comprehensive biotechnology. 2nd ed. Vol. 4. Dordrecht: Elsevier; 2011. p. 165–176.10.1016/B978-0-08-088504-9.00243-9
- Roy H, Andrews TJ. Rubisco: assembly and mechanism. In: Leegood RC, Sharkey TD, von Caemmerer S, editors. Photosynthesis: physiology and metabolism. Dordrecht: Kluwer, Elsevier; 2000. p. 53–83.10.1007/0-306-48137-5
- Jordan DB, Ogren WL. Species variation in the specificity of ribulose biphosphate carboxylase/oxygenase. Nature. 1981;291(5815):513–515.10.1038/291513a0
- Uemura K, Anwaruzzaman MS, Yokota A. Ribulose-1,5-bisphosphate carboxylase/oxygenase from thermophilic red algae with a strong specificity for CO2 fixation. Biochem Biophys Res Commun. 1997;233(2):568–571.10.1006/bbrc.1997.6497
- Wang Y, Stessman DJ, Spalding MH. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: how Chlamydomonas works against the gradient. Plant J. 2015;82(3):429–448.10.1111/tpj.12829
- Andrews TJ, Whitney SM. Manipulating ribulose bisphosphate carboxylase/oxygenase in the chloroplasts of higher plants. Arch Biochem Biophys. 2003;414(2):159–169.
- Lorimer GH, Miziorko HM. Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+. Biochemistry. 1980;19(23):5321–5328.10.1021/bi00564a027
- Taylor TC, Andersson I. Structural transitions during activation and ligand binding in hexadecameric Rubisco inferred from the crystal structure of the activated unliganded spinach enzyme. Nat Struct Biol. 1996;3(1):95–101.10.1038/nsb0196-95
- Cleland WW, Andrews TJ, Gutteridge S, et al. Mechanism of Rubisco: the carbamate as general base. Chem Rev. 1998;98(2):549–562.10.1021/cr970010r
- Habash DZ1, Parry MA, Parmar S, et al. The regulation of component processes of photosynthesis in transgenic tobacco with decreased phosphoribulokinase activity. Photosynth Res. 1996;49(2):159–167.10.1007/BF00117666
- Somerville CR, Portis AR, Ogren WL. A mutant of Arabidopsis thaliana which lacks activation of RuBp carboxylase in vivo. Plant Physiol. 1982;70(2):381–387.10.1104/pp.70.2.381
- Portis AR Jr, Li C, Wang D, et al. Regulation of Rubisco activase and its interaction with Rubisco. J Exp Bot. 2008;59(7):1597–1604.
- Yamori W, Nagai T, Makino A, The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ. 2011;34(5):764–777.
- Makino A, Sage RF. Temperature response of photosynthesis in transgenic rice transformed with ‘sense’ or ‘antisense’ rbcS. Plant Cell Physiol. 2007;48(10):1472–1483.10.1093/pcp/pcm118
- Kubien DS, Sage RF. The temperature response of photosynthesis in tobacco with reduced amounts of Rubisco. Plant Cell Environ. 2008;31(4):407–418.10.1111/j.1365-3040.2008.01778.x
- Fukayama H, Ueguchi C, Nishikawa K, et al. Overexpression of Rubisco activase decreases the photosynthetic CO2 assimilation rate by reducing Rubisco content in rice leaves. Plant Cell Physiol. 2012 Jun;53(6):976–986.10.1093/pcp/pcs042
- McCann N, Phan D, Wang X, et al. Kinetics and mechanism of carbamate formation from CO2(aq), carbonate species, and monoethanolamine in aqueous solution. J Phys Chem A. 2009;113(17):5022–5029.10.1021/jp810564z
- Vater S, Salnikow J, Kleinkauf H. A fluorimetric study of substrate and effector binding of D-ribulose-1,5-biphosphate carboxylase/oxygenase from spinach. Biochem Biophys Res Commun. 1977;74(4):1618–1625.10.1016/0006-291X(77)90628-3
- Stotz M, Mueller-Cajar O, Ciniawsky S, et al. Structure of green-type Rubisco activase from tobacco. Nat Struct Mol Biol. 2011;18(12):1366–1370.10.1038/nsmb.2171
- Mueller-Cajar O, Stotz M, Wendler P, et al. Structure and function of the AAA+ protein CbbX, a red-type Rubisco activase. Nature. 2011;479(7372):194–199.10.1038/nature10568
- Salvucci ME, Portis AR Jr, Ogren WL, A soluble chloroplast protein catalyzes ribulosebisphosphate carboxylase/oxygenase activation in vivo. Photosynth Res. 1985;7(2):193–201.
- Eckardt NA1, Snyder GW, Portis AR Jr, et al, Growth and photosynthesis under high and low irradiance of Arabidopsis thaliana antisense mutants with reduced ribulose-1,5-bisphosphate carboxylase/oxygenase activase content. Plant Physiol. 1997;113(2):575–586.10.1104/pp.113.2.575
- Mate CJ, von Caemmerer S, Evans JR, et al. The relationship between CO2-assimilation rate, Rubisco carbamylation and Rubisco activase content in activase-deficient transgenic tobacco suggests a simple model of activase action. Planta. 1996;198(4):604–613.10.1007/BF00262648
- Hammond ET, Andrews TJ, Mott KA, et al. Regulation of Rubisco activation in antisense plants of tobacco containing reduced levels of Rubisco activase. Plant J. 1998;14(1):101–110.10.1046/j.1365-313X.1998.00103.x
- Lan Y, Mott KA. Determination of apparent km values for ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase using the spectrophotometric assay of Rubisco activity. Plant Physiol. 1991;95(2):604–609.10.1104/pp.95.2.604
- McCurry SD, Pierce J, Tolbert NE, et al. On the mechanism of effector-mediated activation of ribulose bisphosphate carboxylase/oxygenase. J Biol Chem. 1981 Jul 10;256(13):6623–6628.
- Gutteridge S, Parry MA, Schmidt CN. The reactions between active and inactive forms of wheat ribulosebisphosphate carboxylase and effectors. Eur J Biochem. 1982;126(3):597–602.10.1111/ejb.1982.126.issue-3
- Jordan DB, Chollet R, Ogren WL. Binding of phosphorylated effectors by active and inactive forms of ribulose- 1, s, -bisphosphate carboxylase. Biochemistry. 1983;22(14):3410–3418.10.1021/bi00283a017
- Zhu G, Jensen RG. Fallover of ribulose 1,5-bisphosphate carboxylase/oxygenase activity : decarbamylation of catalytic sites depends on pH. Plant Physiol. 1991;97(4):1354–1358.10.1104/pp.97.4.1354
- Badger MR, Lorimer GH. Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1981;20(8):2219–2225.10.1021/bi00511a023
- Matsumura H, Mizohata E, Ishida H, et al. Crystal structure of rice Rubisco and implications for activation induced by positive effectors NADPH and 6-phosphogluconate. J Mol Biol. 2012;422(1):75–86.10.1016/j.jmb.2012.05.014
- Heineke D, Riens B, Grosse H. Redox transfer across the inner chloroplast envelope membrane. Plant Physiol. 1991 Apr;95(4):1131–1137.10.1104/pp.95.4.1131
- Buchanan BB. Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch Biochem Biophys. 1991;288(1):1–9.10.1016/0003-9861(91)90157-E
- Badger MR, Sharkey TD, von Caemmerer S. The relationship between steady-state gas exchange of bean leaves and the levels of carbon-reduction-cycle intermediates. Planta. 1984 Mar;160(4):305–313.10.1007/BF00393411
- Dumas R, Joyard J, Douce R. Effect of sulphate on glutamate synthesis by intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1989;259(3):769–774.10.1042/bj2590769
- Martin W, Scheibe R, Schnarrenberger C, The Calvin cycle and its regulation. In Leegood RC, Sharkey TD, von Caemmerere S, editors. Photosynthesis: physiology and metabolism. Alphen aan den Rijn: Kluwer; 2000. p. 9–51, 10.1007/0-306-48137-5
- Price GD, Evans JR, von Caemmerer S, et al. Specific reduction of chloroplast glyceraldehyde-3-phosphate dehydrogenase activity by antisense RNA reduces CO2 assimilation via a reduction in ribulose bisphosphate regeneration in transgenic tobacco plants. Planta. 1995;195(3):369–378.10.1007/BF00202594
- Banks FM, Driscoll SP, Parry MA, et al. Decrease in phosphoribulokinase activity by antisense RNA in transgenic tobacco. relationship between photosynthesis, growth, and allocation at different nitrogen levels. Plant Physiol. 1999;119(3):1125–1136.10.1104/pp.119.3.1125
- Haake V, Zrenner R, Sonnewald U, et al. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J. 1998;14(2):147–157.10.1046/j.1365-313X.1998.00089.x
- Quick WP, Schurr U, Scheibe R. Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with “antisense” rbcS : I. Impact on photosynthesis in ambient growth conditions. Planta. 1991;183(4):542–554.
- Hudson GS1, Evans JR, von Caemmerer S, Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase content by antisense RNA reduces photosynthesis in transgenic tobacco plants. Plant Physiol. 1992;98(1):294–302.10.1104/pp.98.1.294
- Kossmann J, Uwe Sonnewald U, Willmitzer L. Reduction of the chloroplastic fructose-1,6-bisphosphatase in transgenic potato plants impairs photosynthesis and plant growth. Plant J. 1994;6(5):637–650.10.1046/j.1365-313X.1994.6050637.x
- Harrison EP, Olcer H, Lloyd JC, et al. Small decreases in SBPase cause a linear decline in the apparent RuBP regeneration rate, but do not affect Rubisco carboxylation capacity. J Exp Bot. 2001;52(362):1779–1784.10.1093/jexbot/52.362.1779
- Tamoi M, Murakami A, Takeda T, et al. Acquisition of a new type of fructose-1,6-bisphosphatase with resistance to hydrogen peroxide in cyanobacteria: molecular characterization of the enzyme from Synechocystis PCC 6803. Biochim Biophys Acta. 1998;1383(2):232–244.10.1016/S0167-4838(97)00208-2
- Miyagawa Y, Tamoi M, Shigeoka S. Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat Biotechnol. 2001 Oct;19(10):965–969.10.1038/nbt1001-965
- Yabuta Y, Tamoi M, Yamamoto K, et al. Molecular design of photosynthesis-elevated chloroplasts for mass accumulation of a foreign protein. Plant Cell Physiol. 2008;49(3):375–385.10.1093/pcp/pcn014
- Portis AR1, Salvucci ME, Ogren WL, Activation of ribulosebisphosphate carboxylase/cxygenase at physiological CO2 and ribulosebisphosphate concentrations by Rubisco activase. Plant Physiol. 1986;82(4):967–971.10.1104/pp.82.4.967
- Yokota A. Ribulose bisphosphate-induced, slow conformational changes of spinach ribulose bisphosphate carboxylase cause the two types of inflections in the course of its carboxylase reaction. J Biochem. 1991;110(2):246–252.10.1093/oxfordjournals.jbchem.a123565
- Yokota A, Higashioka M, Wadano A. Cooperative binding of carboxyarabinitol bisphosphate to the regulatory sites of ribulose bisphosphate carboxylase/oxygenase from spinach. J Biochem. 1991;110(2):253–256.10.1093/oxfordjournals.jbchem.a123566
- Yokota A, Higashioka M, Wadano A. Regulation of the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase through cooperative binding of 6-phosphogluconate to its regulatory sites. Eur J Biochem. 1992;208(3):721–727.10.1111/ejb.1992.208.issue-3
- Perchorowicz JT, Jensen RG. Photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings : regulation by CO2 and O2. Plant Physiol. 1983;71(4):955–960.10.1104/pp.71.4.955
- Takahara K, Kasajima I, Takahashi H. Metabolome and photochemical analysis of rice plants overexpressing Arabidopsis NAD kinase gene. Plant Physiol. 2010;152(4):1863–1873.10.1104/pp.110.153098
- Cardona T, Murray JW, Rutherford AW. Origin and evolution of water oxidation before the last common ancestor of the cyanobacteria. Mol Biol Evol. 2015;32(5):1310–1328.10.1093/molbev/msv024
- Ezaki S, Maeda N, Kishimoto T, et al. Presence of a structurally novel type ribulose-bisphosphate carboxylase/oxygenase in the hyperthermophilic archaeon, Pyrococcus kodakaraensis KOD1. J Biol Chem. 1999 Feb 19;274(8):5078–5082.10.1074/jbc.274.8.5078
- Tabita FR. Microbial ribulose 1,5-bisphosphate carboxylase/oxygenase: a different perspective. Photosynth Res. 1999;60(1):1–28.10.1023/A:1006211417981
- Sato T, Atomi H, Imanaka T. Archaeal Type III RuBisCOs function in a pathway for AMP metabolism. Science. 2007;315(5814):1003–1006.10.1126/science.1135999
- Hanson TE, Tabita FR. A ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO)-like protein from Chlorobium tepidum that is involved with sulfur metabolism and the response to oxidative stress. Proc Natl Acad Sci USA. 2001;98(8):4397–4402.10.1073/pnas.081610398
- Ashida H, Saito Y, Kojima C. A functional link between RuBisCO-like protein of bacillus and photosynthetic RuBisCO. Science. 2003;302(5643):286–290.10.1126/science.1086997
- Ashida H, Saito Y, Nakano T, et al. RuBisCO-like proteins as the enolase enzyme in the methionine salvage pathway: functional and evolutionary relationships between RuBisCO-like proteins and photosynthetic RuBisCO. J Exp Bot. 2008;59(7):1543–1554.
- Tabita FR, Hanson TE, Li H, et al. Function, structure, and evolution of the rubisco-like proteins and their RubisCO homologs. Microbiol Mol Biol Rev. 2007 Dec;71(4):576–599.10.1128/MMBR.00015-07
- Mueller-Cajar O, Badger MR. New roads lead to Rubisco in archaebacteria. BioEssays. 2007 Aug;29(8):722–724.10.1002/bies.v29:8
- Kono T, Mehrotra S, Endo C, et al. A RuBisCO-mediated carbon metabolic pathway in methanogenic archaea. Nat Commun. 2017;8:14007. doi:10.1038/ncomms14007.
- Kasting JF, Pollack JB, Effects of high CO2 levels on surface temperature and atmospheric oxidation state of the early Earth. J Atmos Chem. 1984(4);1:403–428.10.1007/BF00053803
- Bartoschek S, Vorholt JA, Thauer RK, et al. N-Carboxymethanofuran (carbamate) formation from methanofuran and CO2 in methanogenic archaea: Thermodynamics and kinetics of the spontaneous reaction. Eur J Biochem. 2000;267(11):3130–3138.10.1046/j.1432-1327.2000.01331.x
