Abstract
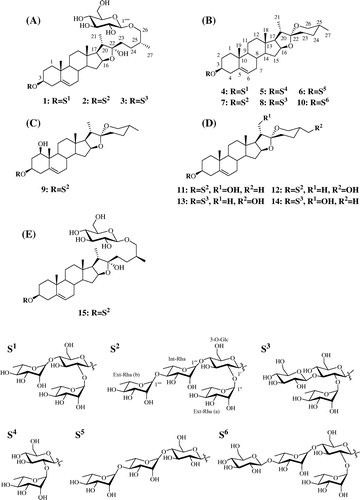
Fifteen steroidal saponins 1–15, which include 4 furostanol glycosides 1–3 and 15, and 11 spirostanol glycosides 4–14, were isolated from the tubers and leaves of lesser yam (Dioscorea esculenta, Togedokoro). Their structures were identified by nuclear magnetic resonance and liquid chromatography mass spectroscopy. Four steroidal saponins 9, 11, 14, and 15 were found to be novel compounds.
Steroidal saponins from Dioscorea esculenta.

Dioscorea esculenta (Togedokoro) is known as a lesser yam of Dioscorea species, and is widely distributed and cultivated for foods in parts of Southern Asia and the Pacific. The tubers of D. esculenta have been used traditionally as a medicine in the treatment of various diseases.Citation1) The edible tubers of Dioscorea species have been reported to contain high amounts of steroidal saponins (sometimes with a yield >2%) as the functional compounds.Citation2) Steroidal saponins are conjugated with several sugars at the C3 and/or C26 hydroxy group of steroid, and they are natural surfactants with various biological activities, including hemolytic, cytotoxic, anti-inflammatory, antifungal, and antibacterial properties.Citation3) Based on the ring structure, the steroidal moiety is classified into three types; furostane of a pentacyclic ABCDE-ring system with a side chain at C22, spirostane of a hexacyclic ABCDEF-ring system, and pregnane of a tetracyclic ABCD-ring system.Citation2,4) Over 50 steroidal saponins have been reported from various Dioscorea species,Citation2) in particular, the tubers of Dioscorea spp. are known to contain furostane-type and spirostane-type glycosides such as protodioscin and dioscin, respectively.Citation5) These steroidal saponins from Dioscorea spp. are valuable starting materials for the synthesis of pharmaceutical steroidal drugs such as anti-inflammatory, androgenic, estrogenic, and contraceptive drugs.Citation6,7)
However, to our knowledge, the structures of steroidal saponins from D. esculenta have not yet been fully investigated. In the present study, 15 steroidal saponins including 4 novel compounds were isolated from tubers and leaves of D. esculenta, and their structures were determined by nuclear magnetic resonance (NMR) and liquid chromatography mass spectroscopy (LC-MS).
Materials and methods
Materials and chemicals
The freeze-dried rhizomes powder of D. esculenta was purchased from Takara Bio Inc. (Otsu, Japan). Acetonitrile (MeCN) and methanol (MeOH) which were used for analyses by a high-performance liquid chromatography (HPLC) were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Ethanol (EtOH), n-hexane, ethyl acetate (EtOAc), and 1-butanol (BuOH), which were used for extraction and solvent fractionation, were obtained from Kanto Chemical Co., Inc. (Tokyo, Japan).
Extraction and solvent fractionation
The tuber powder (1.2 kg) of D. esculenta was extracted with 70% EtOH (4 L) for 2 h at 80 °C. Then, the mixture was filtered under vacuum through No. 2 filter paper (Advantec, Tokyo, Japan), and the residue was repeatedly extracted with 70% EtOH (4 L). The solutions were combined and concentrated in a vacuum at 38 °C. The extract (91.0 g) was suspended in water (700 mL) and partitioned with n-hexane (700 mL × 3), EtOAc (700 mL × 3), and H2O saturated-BuOH (700 mL × 3). Each fraction was spotted on silica gel thin-layer chromatography (TLC; silica gel 60 F254, 0.25 mm thickness; Merck, Darmstadt, Germany) and developed using a mixture of CHCl3/MeOH/H2O (8:4.5:1, v/v/v). The spots were detected by spraying with anisaldehyde solution in EtOH. LC-MS analysis of each fraction was also carried out using as following conditions. LC was carried out using an Acquity UPLC system (Waters Corp., Milford, USA) with an Acquity UPLC HSS T3 column (1.8 μm, 2.1 × 100 mm, Waters Corp.) protected by a guard column of the same phase at 30 °C. Samples (5 μL) were injected onto a column and the steroidal saponins were eluted from the column with a gradient elution system consisting of 0.1% aqueous formic acid (A) and 100% MeCN (B) as mobile phases. The gradient was started at 10% B and linearly increased to 55% B for 30 min, followed by a linear increase to 75% B for 5 min at a flow rate of 0.2 mL/min. The MS instrument used was a Synapt MS system (Waters Corp.). Samples were analyzed in a positive electron spray ionization (ESI) mode and the data were acquired from 100 to 1500 Da. The MS source temperature was 120 °C and the desolvation temperature was 450 °C with desolvation gas flow set at 900 L/h. The capillary voltage was 3 kV. The cone voltage was 30 V and the collision energy was 20 eV. The instrument was controlled by Masslynx software (Waters Corp.).
The leaf (80.0 g) of D. esculenta was extracted with 70% EtOH (1 L) under the same condition with preparation of powder extract. And then the mixture was filtrated, the solutions were combined and concentrated in a vacuum at 38 °C. The extract (5.9 g) was suspended in water (100 mL) and partitioned with n-hexane (100 mL × 3), EtOAc (100 mL × 3), and H2O-saturated BuOH (100 mL × 3). The fractions were checked with TLC and LC-MS with same condition as above.
Isolation and purification of furostane-type steroidal saponins
At the isolation of furostane-type steroidal saponins from tuber powder extract, the steroidal saponins were mostly detected in BuOH layer (15.3 g). The BuOH layer (3.1 g) was subjected to C18 solid phase extraction (SPE, Sep-Pak Vac 35 cc, 10 g, Waters, Ireland) and eluted with H2O/EtOH (100:0, 80:20, 60:40, 50:50, 40:60, 20:80, 0:100; v/v, step-wide system, 70 mL) as elution solvents. To avoid methylation of furostane-type steroidal saponins by MeOH used as elution solvent, H2O/EtOH was used as elution solvent for isolation of furostane-type steroidal saponins. Each fraction was subjected to TLC and LC-MS analysis. The fraction (434.9 mg) eluted with H2O/EtOH 60:40 contained most of steroidal saponins was separated with a preparative-scale HPLC (Shimadzu, Kyoto, Japan), operated by the following system: ODS column (5C18-PAQ, 20 × 250 mm, Nacalai) protected by a guard column (5C18-PAQ, 10 × 10 mm, Nacalai) of an isocratic system of H2O/MeCN (74:26, v/v, HPLC-1) at 30 °C. Flow rate was 5.0 mL/min and eluents were monitored at 203 nm. Three fractions, E1 (tR 45.4 min, 54.6 mg), E2 (tR 48.2 min, 119.7 mg), and E3 (tR 52.0 min, 27.2 mg) were obtained. The obtained E1 and E2 were further purified by preparative HPLC using an isocratic system of H2O/MeCN (75:25, v/v, HPLC-2) for isolation of compound 1 (tR 71.5 min, 13.8 mg) and 2 (tR 79.6 min, 37.5 mg). E3 was further purified by preparative HPLC using an isocratic system of HPLC-1 conditions for isolation of compound 3 (tR 61.8 min, 13.9 mg).
At the isolation of furostane-type steroidal saponins from leaf extract (5.9 g), steroidal saponins were mostly detected in BuOH layer (1.5 g) obtained after solvent fractionation of the leaf extract. The BuOH layer was subjected to C18 SPE using H2O/EtOH (100:0, 80:20, 60:40, 50:50, 20:80, 0:100; v/v, step-wide system, 70 mL) as elution solvents to afford. The fraction (490.1 mg) eluted with H2O/EtOH 60:40 was purified with preparative HPLC using HPLC-1 conditions. The subfraction (tR 52.5 min, 49.2 mg) thus obtained was further purified using preparative HPLC with HPLC-2 conditions to give compound 15 (tR 65.8 min, 26.4 mg).
Isolation and purification of spirostane-type steroidal saponins
The BuOH layer (12.2 g) obtained from tuber powder extract was subjected to C18 SPE and eluted with H2O/MeOH (100:0, 80:20, 60:40, 40:60, 20:80, 0:100; v/v, step-wide system, 70 mL) as elution solvents. The fraction (1.1 g) eluted with H2O/MeOH 40:60 was separated by silica gel (50 g, 1.9 × 35.5 cm, Wako Pure Chemical Industries, Osaka, Japan) column chromatography using CHCl3/MeOH (90:10, 80:20, 75:25, 60:40, 50:50, and 0:100, v/v). From results of LC-MS and TLC analysis, steroidal saponins were detected in fraction M1-M3 eluted by CHCl3/MeOH 80:20 and 75:25. These fractions were subjected to preparative HPLC using an isocratic system of H2O/MeOH (25:75, v/v, HPLC-3). Compound 4 (tR 82.8 min, 39.1 mg), 5 (tR 89.1 min, 2.9 mg), and 6 (tR 94.8 min, 0.8 mg) from M1 (67.9 mg), compound 7 (tR 79.6 min, 132.0 mg) and 8 (tR 84.2 min, 11.2 mg) from M2 (210.1 mg), and compound 9 (tR 36.1 min, 1.9 mg) and 10 (tR 73.5 min, 2.7 mg) from M3 (100.8 mg) were isolated, respectively. M2-1 (8.6 mg) and M3-1 (12.3 mg) were further purified by preparative HPLC using an isocratic system of H2O/MeCN (70:30, v/v, HPLC-4) to give compound 11 (tR 136.1 min, 6.0 mg) from M2-1, and compound 12 (tR 98.8 min, 3.1 mg), 13 (tR 106.7 min, 0.5 mg) and 14 (tR 148.0 min, 1.0 mg) from M3-1.
GC analysis of the aglycone of 2 and 15
Compounds 2 and 15 (100 μg) were treated with 1 N HCl (300 μL) at 100 °C for 30 min. After cooling, the reaction mixture was extracted three times with EtOAc 300 μL. Then the extract was evaporated to dryness under a vacuum, the residue was trimethylsilylated with TMS-HT (HMDS and TMCS in Anhydrous Pyridine, Tokyo Chemical Industry Co., Ltd.) at 80 °C for 30 min. GC-MS was conducted using a GC-MS-QP2010 Ultra (Shimadzu) with a DB-1MS (30 m × 0.25 mm, 0.25 μm film thickness; J&W Scientific) capillary column. The injection temperature was 250 °C. The column temperature program for analysis was as follows: 180 °C for 1 min, followed by a rise to 280 °C at a rate of 20 °C/min and to 300 °C at a rate of 2 °C/min, and then a hold at 300 °C for 16 min. The carrier gas was He, and the flow rate was 1.0 mL/min; the interface and ion source temperature were 300 and 250 °C, with a splitless injection. MS scan mode with a mass range of m/z 50–700 was used.
Structural analysis of steroidal saponins
NMR spectra were recorded on a Bruker AVANCEIII 600 spectrometer (600 MHz for 1H and 151 MHz for 13C; Bruker BioSpin Corp., Billerica, MA, USA). The chemical shifts are reported in parts per million relative to tetramethylsilane (0.00 ppm) as an internal standard (Tables and ). The assignments of the 1H and 13C signals were based on 1H–1H correlation spectroscopy (COSY), heteronuclear single quantum coherence (HSQC), heteronuclear multiple bond correlation (HMBC), and nuclear Overhauser enhancement and exchange spectroscopy (NOESY) spectra. The isolated compounds were dissolved in C5D5N (Aldrich, USA). ESI Mass spectra were acquired on a LC-MS as mentioned above and high-resolution fast atom bombardment mass spectroscopy (HR-FAB-MS) spectra of 9, 11, 14, and 15, which are novel compounds, were acquired on JEOL JMS-700 spectrometer (Tokyo, Japan) in negative ion mode.
Table 1. 1H (600 MHz) and 13C NMR (151 MHz) data of aglycone moiety of 9, 11, 14, and 15 in C5D5N.
Table 2. 1H (600 MHz) and 13CNMR (151 MHz) data of sugar moiety of 9, 11, 14, and 15 in C5D5N.
(25R)-Spirost-5-ene-1β,3β-diol-3-O-α-L–rhamnopyranosyl-(1 → 4)-α-L-rhamnopyranosyl-(1 → 4)-[α-L-rhamnopyranosyl-(1 → 2)]-β-D-glucopyranoside (9)
Colorless powder. ESI-MS (positive) m/z 1031.2 [M + H]+, 885.3 [M + H – rhamnose (Rha)]+, 739.1 [M + H – 2Rha]+, 593.4 [M + H – 3Rha]+, 431.0 [M + H – 3Rha – glucose (Glc)]+, 413.0 [M + H – 3Rha – Glc – H2O]+, and 395.0 [M + H – 3Rha – Glc – 2H2O]+. HR-FAB-MS (negative) m/z 1029.5272 [M − H]– (calculated for C51H81O21, m/z 1029.5270).
(25R)-Spirost-5-ene-3β,21-diol-3-O-α-L-rhamnopyranosyl-(1 → 4)-α-L-rhamnopyranosyl-(1 → 4)-[α-L-rhamnopyranosyl-(1 → 2)]-β-D-glucopyranoside (11)
Colorless powder. ESI-MS (positive) m/z 1031.6 [M + H]+, 885.5 [M + H – Rha]+, 739.1 [M + H – 2Rha]+, 592.8 [M + H – 3Rha]+, 430.8 [M + H – 3Rha – Glc]+, 412.9 [M + H – 3Rha – Glc – H2O]+, and 394.9 [M + H – 3Rha – Glc – 2H2O]+. HR-FAB-MS (negative) m/z 1029.5270 [M – H]− (calculated for C51H81O21, m/z 1029.5270).
(25R)-Spirost-5-ene-3β,21-diol-3-O-α-L-rhamnopyranosyl-(1 → 2)-[β-D-glucopyranosyl-(1 → 3)]-β-D-glucopyranoside (14)
Colorless powder. ESI-MS (positive) m/z 901.3 [M + H]+, 738.9 [M + H – Glc]+, 593.0 [M + H – Glc – Rha]+, 431.0 [M + H – 2Glc – Rha]+, 412.8 [M + H – 2Glc – Rha –H2O]+, and 394.9 [M + H – 2Glc – Rha – 2H2O]+. HR-FAB-MS (negative) m/z 899.4640 [M – H]− (calculated for C45H71O18, m/z 899.4640).
(25S)-Furost-5-ene-3β,22α,26-triol-3-O-α-L-rhamnopyranosyl-(1 → 4)-α-L-rhamnopyranosyl-(1 → 4)-[α-L-rhamnopyranosyl-(1 → 2)]-β-D-glucopyranosyl 26-O-β-D-glucopyranoside (15)
Colorless powder
ESI-MS (positive) m/z 1177.4 [M + H – H2O]+, 1031.2 [M + H – H2O – Rha]+, 885.6 [M + H – H2O – 2Rha]+, 739.1 [M + H – H2O – 3Rha]+, 577.0 [M + H – H2O – 3Rha – Glc]+, and 414.7 [M + H – H2O – 3Rha – 2Glc]+. HR-FAB-MS (negative) m/z 1193.5955 [M – H]− (calculated for C57H93O26, m/z 1193.5955).
Results and discussion
Fourteen steroidal saponins 1–14 from tuber powders of D. esculenta and 1 steroidal saponin 15 from leaf of D. esculenta were isolated. Among 15 saponins, seven steroidal saponins 1–4, 7, 8, and 15 isolated an amount more than 11 mg were considered to be relatively highly accumulated, while 8 steroidal saponins 5, 6, 9–14 isolated an amount under 6 mg were considered to be minor saponins with a low content.
Based on MS, 1H, and 13C NMR spectra, the isolated three steroidal sapoinins, compound 1, 2, and 3, were identified as protodioscin (1),Citation8) dichotomin (2),Citation9,10) and protogracillin (3),Citation11,12) which are furostane-type saponins. Compound 4, 5, 7, 8, 10, 12, and 13 were identified as dioscin (4),Citation13,14) prosapogenin A (5),Citation15) parrisaponin (7),Citation16) gracillin (8),Citation17,18) (25R)-spirost-5-en-3β-ol-3-O-β-D-glucopyranosyl-(1 → 4)-α-L-rhamnopyranosyl-(1 → 4)-[α-L-rhamnopyranosyl-(1 → 2)]-β-D-glucopyranoside (10),Citation16) (25R)-spirost-5-ene-3β,27-diol-3-O-α-L-rhamnopyranosyl-(1 → 4)-α-L-rhamnopyranosyl-(1 → 4)-[α-L-rhamnopyranosyl-(1 → 2)]-β-D-glucopyranoside (12),Citation19) and (25R)-spirost-5-ene-3β,27-diol-3-O-α-L-rhamnopyranosyl-(1 → 2)-[β-D-glucopyranosyl-(1 → 3)]-β-D-glucopyranoside (13),Citation20,21) which are spirostane-type saponin. Also, compound 6 was estimated as (25R)-spirost-5-en-3β-ol-3-O-α-L-rhamnopyranosyl-(1 → 4)-α-L-rhamnopyranosyl-(1 → 4)-β-D-glucopyranoside of spirostane-type saponin by 1H NMR and ESI-MS data compared with those spectra data reported previously (Fig. ).Citation16,22)
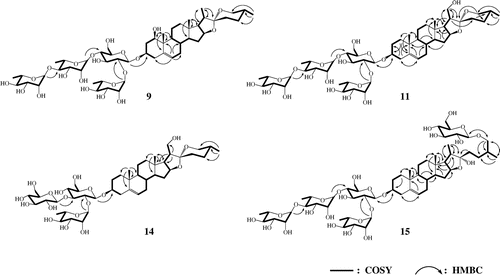
The molecular formula of 9 was determined to be C51H82O21 by negative HR-FAB-MS analysis. The positive ESI-MS spectra of 9 suggested that 9 is a spirostane-type glycoside with four sugar moieties of one glucopyranose and three rhamnopyranose. The 1H NMR (Table ) spectrum of the aglycone moiety of 9 showed signals of four steroid methyl groups (δ 0.70, 0.91, 1.11, and 1.39), one olefinic proton (δ 5.55), two methylene protons bearing an oxygen function (δ 3.52 and 3.60), three methine protons bearing an oxygen function (δ 3.72, 4.04, and 4.54), one hydroxyl proton (δ 6.27). 1H NMR spectrum (Table ) of the sugar moiety of 9 showed signals of two methylene of glucopyranose (δ 4.04 and 4.14), four anomeric protons (δ 5.00, 5.87, 6.32, and 6.44) revealed the presence of glucopyranose and rhamnopyranose, and three methyl groups of rhamnopyranosyl moieties (δ 1.61, 1.62, and 1.74). 1H NMR spectrum of the sugar moiety of 9 resembled these for the structure of 7Citation16) and 12.Citation19) These results suggested that 9 consists of a spirostane-type aglycone with two hydroxy groups and four sugar moieties of a glucopyranose and three rhamnopyranose [Ext-Rha (a), Int-Rha and Ext-Rha (b)]. From the 13C NMR spectrum (Tables and ), the results were further supported by the presence of signals of seven methyl groups of steroid and rhamnopyranosyl moieties (δ 13.75, 15.07, 16.61, 17.36, 18.47, 18.65, and 18.92), two methylene carbons bearing an oxygen function (δ 61.09 and 66.87), three methine carbons bearing an oxygen function (δ 75.00, 77.96, and 81.11), two olefinic carbons (δ 125.22 and 139.08), three quaternary carbon (δ 43.83, 40.23, and 109.28), and four anomeric carbons of sugars (δ 100.47, 102.18, 102.21, and 103.38). As shown in Fig. , the steroid and oligoglycoside moieties were assigned from their proton–proton correlations in the 1H–1H COSY spectrum and the long-range correlations between protons and carbons by the HMBC spectrum. In particular, the observed HMBC cross-peaks (H1α and C19; H19 and C1) indicated that the hydroxyl group was coupled with the C1 of spirostane aglycone, and the configuration was determined as β by the coupling constant (J = 11.6, 6.1, and 4.8 Hz) of H1α and good agreement with those of clintonioside A.Citation23) In the NOESY spectrum, H1α also correlated with H3α and H9α. The HMBC cross-peak between H1′ and C3 proved that the glucopyranose was coupled with C3 of spirostane aglycone. The linkage of the sugar moiety was also determined by the HMBC correlation of the anomeric protons (Fig. ). The stereochemistry of C22 was confirmed by comparison of the chemical shifts of C23 (δ 31.86), C24 (δ 29.30), and C26 (δ 66.87) with those for 22α-O-spirostanol glycosides reported.Citation19,24) On the basis of these results, 9 was determined to be (25R)-spirost-5-ene-1β,3β-diol-3-O-α-L-rhamnopyranosyl-(1 → 4)-α-L-rhamnopyranosyl-(1 → 4)-[α-L-rhamnopyranosyl-(1 → 2)]-β-D-glucopyranoside, a novel steroidal saponin.
The positive ESI-MS spectra of 11 suggested that 11 is a spirostane-type glycoside with two hydroxyl groups and four sugar moieties of one glucopyranose and three rhamnopyranose. The 1H NMR (Table ) spectrum of the aglycone moiety of 11 showed signals of three steroid methyl groups (δ 0.69, 0.93, and 1.05), one olefinic proton (δ 5.33), two methylenes bearing an oxygen function (δ 3.56, 4.03, and 4.21), two methines bearing an oxygen function (δ 3.88 and 4.65) and one hydroxy proton (δ 5.62). The 1H NMR spectrum (Table ) of the sugar moiety showed that the four proton signals (δ 4.97, 5.87, 6.32, and 6.44) of anomeric proton, the methylene proton signals bearing an oxygen function (4.06 and 4.21), and three proton signals (δ 1.61, 1.62, and 1.79) of methyl group were attributed to linkage of one glucopyranose and three rhamnopyranoses with spirostane aglycone. These results suggested that 11 is the spirostanol glycoside which the structure of 11 compared with that of 9 was different only by the location of one hydroxy group. From the 13C NMR spectrum (Tables and ), the results were further supported by the presence of carbon signals assignable to six methyl carbons of steroid and rhamnopyranosyl moieties (δ 16.41, 17.34, 18.47, 18.68, 18.92, and 19.42), three methylene carbons bearing an oxygen function (δ 61.21, 62.51, and 66.90), two methine carbons bearing an oxygen function (δ 78.07 and 81.82), two olefinic carbons (δ 121.84 and 140.83), three quaternary carbon (δ 37.15, 40.73, and 109.29), and four carbons of sugars (δ 100.36, 102.22, 102.23, and 103.37). As shown in Fig. , the HMBC cross-peaks [H17α with C21; H21a and H21b with C17, C20, and C22] were observed. It is indicated that the hydroxy group was coupled with the C21 of spirostane aglycone of 11 unlike that of 9 which was coupled with the C1. The HMBC cross-peaks [H1′ and C3; H1″ and C2′; H1″′ and C4′; H1″″ and C4″′] were also observed. These results proved the presence of 3-O-glucopyranoside and the same sugar moiety with that of 9. In addition, the molecular formula of 11 was determined to be C51H82O21 by negative HR-FAB-MS analysis. Therefore, 11 was determined to be (25R)-spirost-5-ene-3β,21-diol-3-O-α-L-rhamnopyranosyl-(1 → 4)-α-L-rhamnopyranosyl-(1 → 4)-[α-L-rhamnopyranosyl-(1 → 2)]-β-D-glucopyranoside, which is a novel steroidal saponin.
The negative HR-FAB-MS of 14 revealed the molecular formula of C45H72O18. The positive ESI-MS spectra suggested 14 to be a steroidal glycoside, which consists of a spirostane aglycone coupled with two hydroxy groups and three sugars of two glucopyranose and one rhamnopyranose. The 1H NMR (Tables and ) spectrum showed signals (δ 0.69, 0.93, 1.06, 3.55, 3.97, 4.04, 4.21, 4.66, 5.35, and 5.62) of 14 very similar to those of 11, which indicated the presence of the same aglycone with that of 11. And the proton signals (δ 1.78, 4.97, 5.14, and 6.42) indicated the presence of two glucopyranose and one rhamnopyranose. 1H NMR spectrum of the sugar moiety of 14 resembled these for the structure of 8.Citation17,18) From the 13C NMR spectrum (Tables and ) of 14, the results were further supported by the presence of signals of four methyl carbons of steroid and rhamnopyranosyl moieties (δ 16.41, 17.34, 18.74, and 19.42), four methylene carbons bearing an oxygen function [δ 62.45 (2C), 62.52, and 66.90], two methine carbons bearing an oxygen function (δ 77.68 and 81.83), two olefinic carbons (δ 121.89 and 140.79), three quaternary carbon (δ 37.17, 40.74, and 109.29), and three anomeric carbons of sugars (δ 99.98, 102.28, and 104.60). As shown in Fig. , the HMBC correlation between H1′ and C3 indicated that the sugar moiety, two glucopyranoses and one rhamnopyranose, was coupled with the C3 of spirostane aglycone of 14, and the linkage of the sugar moiety was supported by the HMBC cross-peaks [H1″ and C2′; H1″′ and C3′]. Therefore, 14 was determined to be (25R)-spirost-5-ene-3β,21-diol-3-O-α-L-rhamnopyranosyl-(1 → 2)-[β-D-glucopyranosyl-(1 → 3)]-β-D-glucopyranoside, which is a novel compound.
From the positive ESI-MS spectra of 15, a frustane-type glycoside with two glucopyranoses and three rhamnopyranoses was suggested. The 1H NMR (Table ) spectrum of the aglycone moiety of 15 showed signals of three steroid methyl groups (δ 0.90, 1.04, and 1.07), one olefinic proton (δ 5.31), two methylenes bearing an oxygen function (δ 3.49 and 4.10), two methines bearing an oxygen function (δ 3.89 and 4.95), one hydroxy proton (δ 6.69). The 1H NMR (Table ) spectrum of 15 showed the signals that four methylenes of glycopyranose (δ 4.06, 4.21, 4.41, and 4.57), five anomeric protons (δ 4.84, 4.97, 5.87, 6.32, and 6.44) attributable to a linkage of glucopyranose and rhamnopyranose, and three methyl groups of rhamnopyranosyl moieties (δ 1.61, 1.62, and 1.79). These results suggested that 15 resembles to the structure of 2Citation9,10) which consists of furostanol and sugar moieties of two glucopyranoses and three rhamnopyranoses. However, the proton signals of H26 (δ 3.49 and 4.10) and H27 (δ 1.04) of 15 showed different chemical shift with that of H26 (δ 3.63 and 3.96) and H27 (δ 1.00) of 2. The hydrolysis of 2 and 15 liberated diosgenin of 25(R) configuration and yamogenin of 25(S) configuration which were identified by GC-MS with comparison of authentic compounds, respectively. So, it was thought that 15 had the structure of 25(S) configuration, which has a different isomeric structure with 2 assigned to the 25(R) configuration in the aglycone moiety. From the 13C NMR spectrum (Tables and ), the results were further supported by the presence of signals of seven methyl carbons of steroid and rhamnopyranosyl moieties (δ 16.50, 16.50, 17.46, 18.47, 18.69, 18.92, and 19.43), two methylene carbons bearing an oxygen function (δ 61.21 and 62.82), two methine carbons bearing an oxygen function (δ 78.09 and 81.14), two olefinic carbons (δ 121.88 and 140.79), three quaternary carbon (δ 37.16, 40.80, and 110.68), and five anomeric carbons of sugars (δ 100.38, 102.21, 102.23, 103.37, and 105.19). The chemical shift of these carbon signals was observed very similar to that of 25(S) type aglycone of previously identified steroidal saponin.Citation25–27) As shown in Fig. , the HMBC cross-peaks [H1′ and C3; H6″″′ and C26] proved the presence of 3-O-glucopyranoside and 26-O-glucopyranoside. And the same sugar moiety with that of 2 supported by the HMBC correlations [H1′′ and C2′; H1″′ and C4′; H1″″ and C4″′]. In addition, the molecular formula of 15 was determined to be C57H94O26 by negative HR-FAB-MS. On the basis of this evidence, 15 was determined to be (25S)-furost-5-ene-3β,22α,26-triol-3-O-α-L-rhamnopyranosyl-(1 → 4)-α-L-rhamnopyranosyl-(1 → 4)-[α-L-rhamnopyranosyl-(1 → 2)]-β-D-glucopyranosyl 26-O-β-D-glucopyranoside, a novel compound.
Conclusions
Fifteen steroidal saponins 1–15 were isolated from tuber powder and leaf of D. esculenta, and their structures were determined by NMR and MS. Compound 1–3, 15 were furostanol glycosides, and compound 4–14 were spirostanol glycosides. Among them 9, 11, 14, and 15 were determined to be novel compounds. This is the first isolation of 1–15 from D. esculenta, though 1–5, 7, and 8 have already been isolated from Dioscorea spp.,Citation28–30) and 6, 10, 12, and 13 have also been isolated from various plantsCitation8,20–22) The present study would be very useful to investigate the characteristics of saponins in Dioscorea spp..
Author contributions
H.J. Lee and M. Mizutani designed the experiment. H.J. Lee and B. Watanabe performed the experiments and wrote the manuscript. All authors reviewed and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was supported by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN) in Japan.
Supplementary material
The supplemental material for this paper is available at https://doi.org/10.1080/09168451.2017.1381016.
Supplemental_data_Lee_et_170913_revised.docx
Download MS Word (6.4 MB)Acknowledgements
The Bruker AVANCEIII 600 NMR spectrometer and JOEL JMS-700 mass spectrometer in the Joint Usage/Research Center (JURC) at Institute for Chemical Research, Kyoto University.
References
- Olayemi JO, Ajaiyeoba EO. Anti-inflammatory studies of yam (Dioscorea esculenta) extract on wistar rats. Afr J Biotechnol. 2007;6:1913–1915.
- Sautour M, Mitaine-Offer AC, Lacaille-Dubois MA. The Dioscorea genus: a review of bioactive steroid saponins. J Nat Med. 2007;61:91–101.10.1007/s11418-006-0126-3
- Sparg SG, Light ME, Van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004;94:219–243.10.1016/j.jep.2004.05.016
- Wang Y, Gao W, Li X, et al. Chemotaxonomic study of the genus Paris based on steroidal saponins. Biochem Syst Ecol. 2013;48:163–173.10.1016/j.bse.2012.12.011
- Avula B, Wang YH, Ali Z, et al. Chemical fingerprint analysis and quantitative determination of steroidal compounds from Dioscorea villosa, Dioscorea species and dietary supplements using UHPLC-ELSD. Biomed Chromatogr. 2014;28:281–294.10.1002/bmc.3019
- Lv LL, Zheng L, Dong D, et al. Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: a potential new drug for treatment of glioblastoma multiforme. Food Chem Toxicol. 2013;59:657–669.10.1016/j.fct.2013.07.012
- Penga Y, Yang ZH, Wang YX, et al. Pathways for the steroidal saponins conversion to diosgenin during acid hydrolysis of Dioscorea zingiberensis C. H. Wright. Chem Eng Res Des. 2011;89:2620–2625.10.1016/j.cherd.2011.06.008
- Yoshikawa M, Xu FM, Morikawa T, et al. Medicinal flowers. XII. New spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chem Pharm Bull. 2007;55:308–316.10.1248/cpb.55.308
- Munday SC, Wilkins AL, Miles CO, et al. Isolation and structure elucidation of dichotomin, a furostanol saponin implicated in hepatogenous photosensitization of sheep grazing Panicum dichotomiflorum. J Agric Food Chem. 1993;41:267–271.10.1021/jf00026a025
- Lee ST, Mitchell RB, Wang ZR, et al. Isolation, characterization, and quantification of steroidal saponins in switchgrass (Panicum virgatum L.). J Agric Food Chem. 2009;57:2599–2604.10.1021/jf803907y
- Chen SX, Snyder JK. Diosgenin-bearing, molluscicidal saponins from Allium vineale: an NMR approach for the structural assignment of oligosaccharide units. J Org Chem. 1989;54:3679–3689.10.1021/jo00276a033
- Yin J, Liu Y, Liu ZH, et al. Steroidal glycosides from Dioscorea septemloba and their inhibitory activity on bone resorbing. Asian J Tradit Med. 2006;1:1–4.
- Yang Z, Wong ELM, Shum TYT, et al. Fluorophore-appended steroidal saponin (dioscin and polyphyllin D) derivatives. Org Lett. 2005;7:669–672.10.1021/ol0475616
- Deng SJ, Yu B, Hui YZ, et al. Synthesis of three diosgenyl saponins: dioscin, polyphyllin D, and balanitin 7. Carbohydr Res. 1999;317:53–62.10.1016/S0008-6215(99)00066-X
- Hou SJ, Zou CC, Zhou L, et al. Synthesis of three natural diosgenyl glycosides. J Asian Nat Prod Res. 2006;8:689–696.10.1080/10286020500289170
- Yu H, Yu B, Wu XY, et al. Synthesis of a group of diosgenyl saponins with combined use of glycosyl trichloroacetimidate and thioglycoside donors. J Chem Soc Perkin Trans. 2000;1:1445–1453.10.1039/a909218h
- Zou CC, Hou SJ, Shi Y, et al. The synthesis of gracillin and dioscin: two typical representatives of spirostanol glycosides. Carbohydr Res. 2003;338:721–727.10.1016/S0008-6215(03)00004-1
- Shim SH, Lee SY, Kim JS, et al. Norditerpenoid alkaloids and other components from the processed tubers of Aconitum carmichaeli. Arch Pharm Res. 2005;28:1239–1243.10.1007/BF02978206
- Wu X, Wang L, Wang H, et al. Steroidal saponins from Paris polyphylla var. yunnanensis. Phytochemistry. 2012;81:133–143.
- Kintya PK, Gur’eva AS, Mashchenko NE, et al. Structures of some lilioglycosides from the bulbs of Lilium regale. Chem Nat Compd. 1997;33:658–662.10.1007/BF02249634
- Mimaki Y, Sashida Y, Nakamura O, et al. Steroidal saponins from the bulbs of Lilium regale and L. henryi. Phytochemistry. 1993;33:675–682.10.1016/0031-9422(93)85472-4
- Yu H, Han XW, Liu XM, et al. NMR studies on synthesized diosgenyl saponin analogs. Magn Reson Chem. 2000;38:704–706.10.1002/(ISSN)1097-458X
- Mimaki Y, Watanabe K. Clintoniosides A-C, new polyhydroxylated spirostanol glycosides from the rhizomes of Clitonia udensis. Helv Chim Acta. 2008;91:2097–2106.10.1002/hlca.v91:11
- Agrawal PK, Jain DC, Gupta RK, et al. Carbon-13 NMR spectroscopy of steroidal sapogenins and steroidal saponin. Phytochemistry. 1985;24:2479–2496.10.1016/S0031-9422(00)80653-6
- Asami A, Hirai Y, Shoji J. Studies on the constituents of palmae plnats. VI. Steroid saponins and flavonoids of leaves of Phoenix canariensis hort. Ex Chabaud, P. humilis Royle var. hanceana Becc., P. dactylifera L., and Licuala spinose Wurmb. Chem Pharm Bull. 1991;39:2053–2056.10.1248/cpb.39.2053
- Challinor VL, Piacente S, De Voss JJ. NMR assignment of the absolute configuration of C-25 in furostanol steroidal saponins. Steroids. 2012;77:602–608.10.1016/j.steroids.2012.02.002
- Kawano K, Sato H, Sakamura S. Isolation and structure of furostanol saponin in asparagus edible shoots. Agric Biol Chem. 1977;41:1–8.
- Liu HW, Wang SL, Cai B, et al. New furostanol glycosides from the rhizomes of Dioscorea futschauensis R. Kunth. J Asian Nat Prod Res. 2003;5:241–247.10.1080/1028602031000105849
- Sautour M, Mitaine-Offer AC, Miyamoto T, et al. A new steroidal saponin from Dioscorea cayenensis. Chem Pharm Bull. 2004;52:1353–1355.10.1248/cpb.52.1353
- Sautour M, Miyamoto T, Lacaille-Dubois MA. Steroidal saponins and flavan-3-ol glycosides from Dioscorea villosa. Biochem Syst Ecol. 2006;34:60–63.10.1016/j.bse.2005.07.007