Abstract
Helminthosporol was isolated from a fungus, Helminthosporium sativum, as a natural plant growth regulator in 1963. It showed gibberellin-like bioactivity that stimulated the growth of the second leaf sheath of rice. After studying the structure–activity relationship between the compound and some synthesized analogs, it was found that helminthosporic acid (H-acid) has higher gibberellin-like activity and chemical stability than helminthosporol. In this study, we showed that (1) H-acid displays gibberellin-like activities not only in rice but also in Arabidopsis, (2) it regulates the expression of gibberellin-related genes, (3) it induces DELLA degradation through binding with a gibberellin receptor (GID1), and (4) it forms the GID1-(H-acid)-DELLA complex to transduce the gibberellin signal in the same manner as gibberellin. This work shows that the H-acid mode of action acts as an agonist for gibberellin receptor.
Helminthosporic acid, a synthetic analog of fungus plant growth regulator “helminthosporol”, acts as an agonist for gibberellin receptor.
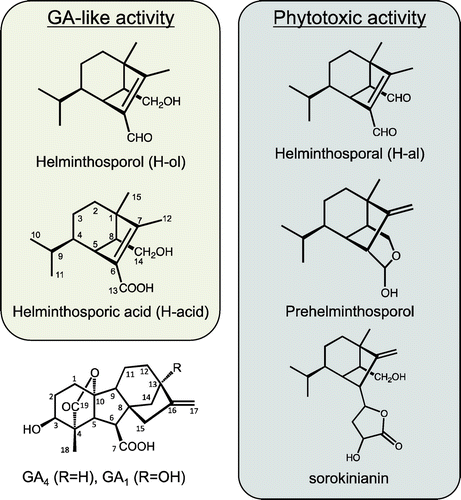
Helminthosporol (H-ol) (Figure ) is a natural sesquiterpenoid isolated from the culture medium of the fungus Cochliobolus sativus, also called Bipolaris somkiniana or Helminthosporium sativum, a pathogenic ascomycete responsible for seedling blight and root rot.Citation1) H-ol is a monoaldehyde sesquiterpene whose chemical structure was initially identified and described as a plant growth regulator, which promotes shoot growth of rice seedlings like gibberellin (GA).Citation2) It has been shown that a dialdehyde analog of H-ol, helminthosporal (H-al) (Figure ), is responsible for inducing plant disease symptoms, especially in wheat.Citation3,4) It has been suggested that prehelminthosporol (Figure ), the precursor of H-al, is responsible for the biological phytotoxicity of the fungus.Citation5–7) Moreover, a compound, sorokinianin (Figure ), has been isolated from the fungus as part of barley seed germination inhibitory activity, and it can be biosynthesized from prehelminthosporol.Citation8) The biosynthesis of these phytotoxic compounds has been studied but is still unclear. To understand the structure–activity relationships of GA activity, Tamura et al. prepared a variety of H-ol-related compounds including helminthosporic acid (H-acid) (Figure ).Citation9) H-acid shows a higher GA-like activity to seedling elongation and seed germination in a wide range of host plants. In addition, the GA-biosynthetic inhibitor, prohexadione, did not inhibit the shoot elongation caused by H-acid.Citation10–13) These results suggest that H-acid acts as a GA mimic in plants but the biochemical mode of action of the GA activity of H-acid has not been clarified.
GAs were first identified in the fungal pathogen of rice, Gibberella fujikuroi.Citation14) Now, GAs comprise a group of over 100 structurally related compounds that are natural products of plants. Only a few GAs have biological activity, such as GA4 and GA1 (Figure ), and they regulate many aspects of growth and development during the plant life cycle. The GA perception system and GA-signaling pathway have been well studied. GA binding allows the GA receptor, GIBBERELLIN INSENSITIVE DWARF1 (GID1), to interact with DELLA, which acts as a repressor of GA responses through affecting gene expression repressors, thereby triggering DELLA destruction through the ubiquitin-proteasome pathway.Citation15–17) Although rice contains one GID1 and one DELLA (SLR1), there are three GID1 orthologs (AtGID1a, b and c) and five DELLA proteins, repressor of ga1–3 (RGA), gibberellic acid-insensitive (GAI), RGA-like 1 (RGL1), RGL2, and RGL3 in Arabidopsis thaliana.Citation18,19)
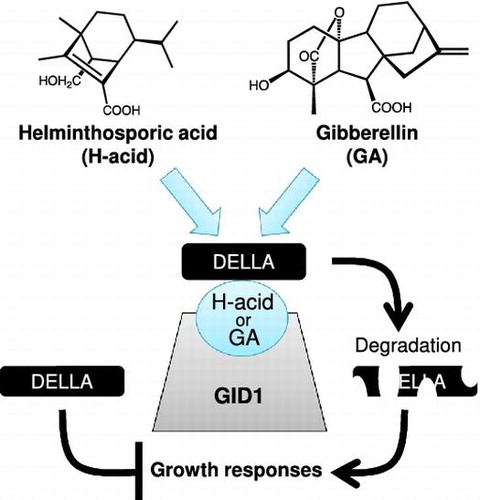
In this paper, we investigated the physiological activity of GA-like natural substance H-acid, which was identified in the 1960s. We demonstrated that H-acid promotes the elongation of seedlings not only in rice but also in Arabidopsis. In addition, H-acid binds to the GID1, forms the GID1-DELLA complex, induces the degradation of DELLA protein, and regulates the expression levels of GA-related genes. Our results show that GA-like substance H-acid acts as an agonist for gibberellin receptor.
Materials and methods
Plant materials and chemicals
Arabidopsis wild-type (Columbia) was used in all experiments. The RGA-GFP line (pRGA::GFP-RGA) was from the Arabidopsis Biological Resource Center. The structure of H-acid was confirmed by NMR at 25 °C on a JEOL ECA 500 spectrometer (JEOL, Akishima, Japan) (Figure S1 and Table S1). The chemical shifts were referenced to tetramethylsilane at 0 ppm. Structural analysis was performed by 1D 1H, and 2D DQF–COSY (double-quantum-filtered correlation spectroscopy), HSQC (heteronuclear single-quantum coherence), HMBC (heteronuclear multiple bond correlation), and NOESY (nuclear overhauser effect spectroscopy) experiments.
Plant growth conditions and bioassay
For micro-drop assay of rice, seeds were sterilized with sodium hypochlorite and germinated in water for 48 h at 30 °C. The seedlings were planted in 0.8% (w/v) agar and incubated for 48 h at 30 °C, under continuous light. In the inhibitory assay, a GA-signaling inhibitor (TSPC) was applied to the agar. The samples were dissolved in 50% (v/v) aqueous acetone and 1 μL of the test compound was applied to the region between the coleoptile and the first leaf of a seedling. Three days later, the length of the second leaf sheath was measured. For expression analysis, germinated seeds were planted in 0.8% (w/v) agar containing GA4, H-acid, or dimethyl sulfoxide (DMSO) as mock control and incubated for 11 days at 30 °C, under continuous light.
For the germination assay, the Arabidopsis seeds were placed on half MS medium containing GA4, H-acid or 0.1% DMSO as mock control, followed by cold treatment for 2 days. Paclobutrazol (PAC) was used for assay. Germination was defined as the protrusion of the radicle after the seeds were transferred from 4 °C to 22 °C under continuous light for 48 h. To measure the hypocotyl length, the seeds were placed on half MS medium. After stratification at 4 °C in the dark for 2 days, the seeds were transferred to 22.5 °C for pulsed-light treatment for 24 h to improve germination. The seeds were then transferred onto new half MS medium containing PAC or PAC and GA4 or PAC and H-acid, and cultivated for 2 days at 25 °C under constant red light. The hypocotyl lengths of seedlings were measured using ImageJ software (National Institutes of Health). To measure the root length, the germinated seeds were transferred onto new half MS medium containing PAC or PAC and GA4 or PAC and H-acid, followed by cultivation for 8 days at 25 °C. The root lengths were scanned and measured with ImageJ.
For the GFP-RGA degradation assay, pRGA::GFP-RGA seeds were placed on half MS. After stratification at 4 °C in the dark for 2 days, the seeds were germinated and cultivated at 22.5 °C for 3 days under constant light (19–23 μmol/m2/s). The seedlings were immersed in liquid half MS containing GA4, H-acid or 0.1% DMSO as mock control for 1 h. GFP florescence was observed under a confocal laser scanning microscope (LSM700, Zeiss).
To prepare the samples for real-time RT-PCR, the germinated seeds were cultivated at 22.5 °C under continuous light for 13 days. The seedlings were transferred to liquid half MS and pre-incubated for 16 h under continuous light. Then, compounds GA4, H-acid or 0.1% DMSO as mock control were added to the medium and cultivated for 1 h at 22.5 °C under continuous light.
Yeast two-hybrid assay
Constructs encoding the GID1s (pGBK-GID1s) and DELLAs (pGAD-DELLAs) of rice and Arabidopsis for the yeast two-hybrid assay were generated as described previously.Citation19) Growth of AH109 transformants was monitored for 3 days with pGBK-GID1s or pGBK-Vec as bait and pGAD-DELLAs as prey on SD–Leu/–Trp/–His/–Ade medium (SD-LWHA) containing GA4, H-acid or 0.1% DMSO as mock control, or containing + His/+Ade medium (SD-LW) as the positive control for yeast growth. 3-Amino-1,2,4-triazole (3-AT) was added to each plate for a final concentration of 1 mM.
Real-time RT-PCR analysis
Total RNA was extracted from frozen plant material using PureLink Plant RNA Reagent (Invitrogen, Life Technologies, CA, USA) for rice and a Total RNA Extraction Mini Kit (RBC Bioscience, Taipei, Taiwan) for Arabidopsis, according to the manufacturer’s instructions. The concentration, purity, and integrity of isolated RNA samples were assessed and confirmed by determining OD260/OD280 ratios, and 500 ng of each RNA sample was used for cDNA synthesis using a ReverTra Ace® qPCR RT Master Mix (TOYOBO, Tokyo, Japan). Quantitative real-time PCR was performed in a 96-well thermocycler (Thermal Cycler Dice® TP 800 Real Time System, Takara, Tokyo, Japan) using the SYBR Premix Ex Taq (Takara). The thermocycler was programmed to run for 30 s at 95 °C, followed by 40 cycles of 5 s at 95 °C and 30 s at 60 °C. Three identically treated replicates of each RNA sample were analyzed to account for biological variation. The primers for PCR, AtGA3ox1, AtEXP1 (encoding expansin1) and AtACT7 (encoding actin7) were used as described previously.Citation20) OsGA20ox2, OsGA2ox4 (Os05g0514600), and the housekeeping gene OsACT1 (encoding actin1) were amplified with the following primers: OsGA20ox2-forward (5′-GCCAATGGGGAGGGTGT-3′) and -reverse (5′-CCATGATCGTCAGCGACA-3′), OsGA2ox4-forward (5′-AGGAGCTTGCTTCCAACAAAG-3′) and -reverse (5′-CAACAGGTTTCCCAACACTG-3′), and OsACT1-forward (5′- TGGCATCTCTCAGCACATTCC-3′) and -reverse (5′-TGTGCACAATGGATGGG-3′).
Molecular docking
Docking simulation analysis of interactions between H-acid and OsGID1 was performed with Chimera and AutoDock Vina based on the data for 3EBL from the Protein Data Bank as described previously.Citation20)
Results
GA-like activity of H-acid in rice and Arabidopsis
GA-like activities of H-acid have been demonstrated in many plants such as rice, grass, and tobacco.Citation10–12) We first confirmed the activity of H-acid using GA-deficient mutant rice Tanginbozu by micro-drop bioassay. After cultivation for 3 days, the length of the second leaf sheath was measured. As shown in Figure (A), the length of the second leaf sheath was elongated by the application of H-acid in a dose-dependent manner similar to GA4.
Figure 2. GA-like activity of H-acid in rice and Arabidopsis.
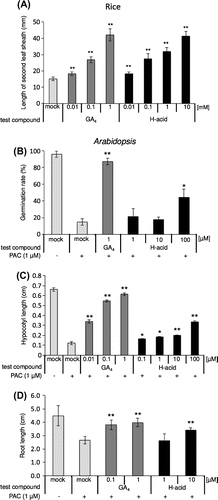
GA signaling has been well studied in Arabidopsis.Citation19,21,22) It is known that GA promotes seed germination in imbibed seeds, hypocotyl elongation, and root elongation, and a GA-biosynthetic inhibitor such as PAC suppressed these activities. We investigated whether H-acid has GA activity in A. thaliana. PAC-inhibited seed germination was restored by the application of higher concentration of H-acid (100 μM) than those in GA4 (Figure (B)). Similar to this, PAC-inhibited hypocotyl growth was restored by the higher concentration of H-acid than that of GA4 (Figure (C)). In addition, root growth was promoted by the application of H-acid, but the activity was lower than that of GA4 (Figure (D)). These results indicate that that H-acid has a very close resemblance in physiological activities to the GAs in A. thaliana, whereas the activities were remarkably weaker than GA4.
H-acid regulates the expression of GA-related genes in the same manner as GA
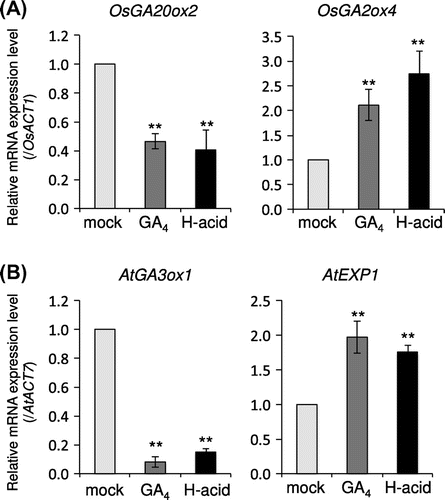
Next, we investigated the effect of H-acid on the GA-related gene expression. In flowering plants, active GA increases the transcript levels of GA-catabolic genes or GA-response genes and decreases the GA-biosynthetic genes.Citation23,24) To determine whether the genes respond to H-acid in a similar manner to GA4, the effects of H-acid on rice GA-biosynthetic and -catabolic gene expression levels were examined. As shown in Figure (A), H-acid suppresses the expression levels of rice GA-biosynthetic gene GA 20-oxidase 2 (OsGA20ox2) and induces the catabolic gene OsGA2ox4. Next, the effects of H-acid on the transcription levels of Arabidopsis GA-related genes were examined. After pre-incubation in liquid half MS medium, GA4 or H-acid were added to the medium and cultivated for 1 h. Similar to rice, H-acid suppresses the expression levels of the GA biosynthesis gene, Arabidopsis GA 3-oxidase 1 (AtGA3ox1), and up-regulates AtEXP1,Citation25) the same as GA4 treatment (Figure (B)). These results strongly suggest that H-acid acts as a GA mimic at the molecular level in planta.
Figure 3. H-acid regulates the expression of GA-related genes in the same manner as GA4.
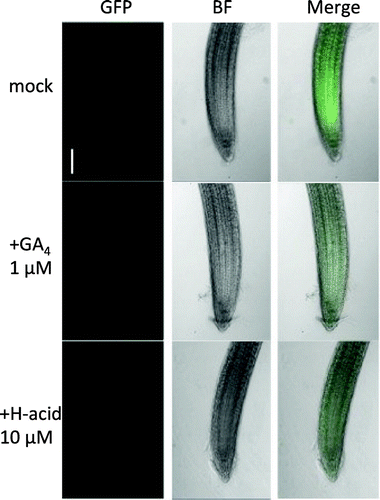
H-acid promotes the degradation of Arabidopsis DELLA protein
The DELLA proteins are conserved repressors of GA signaling that act downstream of the GA receptor to modulate GA-induced growth and development in plants, which are essential for GA-dependent proteasomal degradation of the DELLA proteins. To clarify whether H-acid promotes the degradation of DELLA, a pRGA::GFP-RGA transgenic line (RGA fused to the green fluorescent protein (GFP)) was used. The 5-day-old pRGA::GFP-RGA seedlings were inoculated in liquid half MS medium containing GA4 or H-acid. Similar to GA4 treatment, H-acid treatment leads to the disappearance of fluorescence caused by the degradation of RGA-fused GFP in the primary roots of the seedlings (Figure ). This result suggests that H-acid induces the degradation of DELLA in planta as a GID1 agonist through forming a GID1-(H-acid)-DELLA complex.
H-acid promotes the formation of GID1-DELLA complex
Since H-acid induces the degradation of DELLA, we further investigated whether H-acid shows a GA-like activity to promote interaction between GID1 and DELLA using a yeast two-hybrid assay system.Citation19) The results showed that the yeasts transformed for the OsGID1-SLR1 interaction grew on the medium in the presence of over 0.1 μM H-acid (Figure (A)). As shown in Figure (B), H-acid also promotes the interaction between AtGID1 (GID1a-c) and an AtDELLA protein (RGA) in the presence of over 10 μM H-acid. The interactions depending on H-acid were observed in other GID1s with DELLAs (GAI and RGL1–3) except for RGL1 (Figure S2). Similar to plant bioassay, the GA-like activities promoting interactions between GID1 and DELLA were weaker than GA4 in Arabidopsis. To confirm whether H-acid promotes interaction between GID1s and DELLAs, a GA-signaling inhibitor, TSPC (3-(2-thienylsulfonyl) pyrazine-2-carbonitrile), was used for bioassay.Citation26) In the yeast two-hybrid assay, the interaction between OsGID1 and SLR1 was interrupted by more than 10 μM TSPC in the presence of 1 μM H-acid (Figure (C)). Similarly, the interactions of AtGID1s and RGA mediated by H-acid were interrupted by more than 10 μM TSPC in rice (Figure (D)).
Figure 5. H-acid promotes the formation of GID1-DELLA complex.
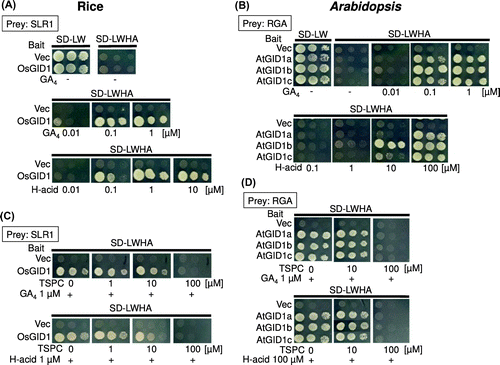
Figure 6. Docking simulation analysis of interaction between H-acid and OsGID1.
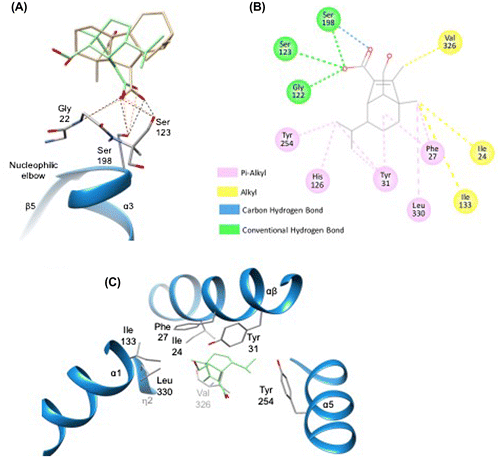
To understand the recognition mechanism of H-acid for GID1, we predicted the GID1 and H-acid complex with automated docking based on the available structure of OsGID1 (Protein Data Bank: 3EBL). Docking simulation of H-acid with OsGID1 showed that the orientation of five- and six-membered rings of H-acid is opposite to those of GA4 and H-acid carboxylate group was located on GA4 carboxylate group of GA4, which is consistent with another hypothesis.Citation27) The carboxylate group in H-acid can introduce hydrogen bonds with hydroxy side chains of Ser 123 and Ser 198, and the main chain NH of Gly 122 in the same manner as GA4 (Figure S3A).Citation16) A further analysis of the simulation results by Discovery StudioCitation28) indicated that H-acid can also form hydrophobic interactions with Ile 24, Phe 27, and Tyr 31 in the αβ lid, and was assumed to facilitate the closing of the N-terminal lid of OsGID1 in the same manner as GA4 (Figure S3B and C). These results indicate that H-acid is recognized to the GID1 receptor and leads to the GID1-DELLA complex, and promotes elongation in the same manner as bioactive GAs.
Discussion
It has been reported that H-ol and its derivatives, including H-acid, act as GA in terms of seed germination and hypocotyl elongation of various plants since the 1960s.Citation2,9–12) However, the GA-signaling pathway was not identified at that time.Citation15) In this work, we showed that H-acid acts as the GA receptor agonist. According to previous reports, we also showed that H-acid promotes the second leaf sheath of rice seedlings (Figure ). Moreover, H-acid rescues the PAC-inhibited Arabidopsis seed germination, and also promotes hypocotyl and root growth (Figure ). These results suggest that H-acid has a functional GA-like activity in both rice and Arabidopsis.
The expression levels of GA-related genes are controlled by feed-forward or -back regulations to modulate GA levels through degradation of DELLA, the repressor of GA signaling. We showed that H-acid regulates the GA-related genes and promotes the degradation of DELLA protein similar to GA4 (Figures and ). This result strongly suggests that H-acid acts as a GA mimic at the molecular level in planta and binds to GID1, which leads to interactions between GID1 and DELLA. Hence, we performed a yeast two-hybrid assay to determine whether H-acid promotes interaction between GID1 and DELLA. In both cases of rice and Arabidopsis, GID1 proteins and DELLA proteins interacted in the presence of H-acid (Figure ). In addition, these interactions were interrupted by the application of GID1 inhibitor TSPC. These results indicate that H-acid acts as the agonist of GID1.
It has been reported that H-ol and H-acid exhibit GA activity with selectivity according to the kinds of plants in contrast to GAs. H-acid promotes the elongation of rice and maize seedlings and cucumber hypocotyls but does not promote elongation of wheat, tomato, and pea.Citation11,12) Our results also indicate the selectivity of H-acid between rice and Arabidopsis, and that GA activity of H-acid compared with GA4 in Arabidopsis was about 10 times less than that of rice in planta and in vitro. Recently, our group reported that the commercial chemical AC94377 is an agonist of GID1 that shares a signaling pathway with GA.Citation20) Although AC94377 has a selectivity specific to the GID1 receptor, H-acid has selectivity to plants. GA3 is now commercially supplied by large-scale fermentation with the fungus G. fujikuroi, and similar to GA3, a natural product H-acid produced by a fungus has a potential as a useful plant growth regulator in agriculture.
The fungus H. sativum infects cereals, particularly wheat and barley, which causes common root rot, and H-al was identified as a phytotoxic sesquiterpenoid compound.Citation3) Interestingly, infected wheat was not elongated because the H-ol, which was produced in the fungus, has no GA activity in wheat. Meanwhile, H-ol promoted rice seedlings but the fungus did not infect the rice as a host. The mode of action of these compounds derived from the fungus has been studied for many years but questions remain; for example, how does the fungus biosynthesize these compounds, and how does the fungus use these compounds for infection of the life cycle? The biochemical mechanisms of these compounds should be elucidated to help improve understanding of the physiological processes and coevolution between the fungus and plants.
Author contributions
S.M., M.K., T.A. and M.N. designed the research. S.M. performed the bioassay, expression analysis, and yeast assay. J.K. performed the homology modeling experiment. S.M. and N.M. wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI [grant number 24380060], [grant number 15H04492 (to MN)], [grant number 16K18693 (to SM)]; the agricultural chemical research foundation (to SM); and the Wada Kunko-kai research foundation (to MN).
Supplemental materials
The supplemental materials for this paper are available at https://doi.org/10.1080/09168451.2017.1381018.
fig_Miyazaki_suppl_R2.pptx
Download MS Power Point (1.2 MB)References
- Ludwig RA. Toxin production by Helminthosporium sativum P.K. & B. and its significance in disease development. Canad J Bot 1957;35:291–304.
- Tamura S, Sakurai A, Kainuma K, et al. Isolation of helminthosporol as a natural plant growth regulator and its chemical structure. Agric Biol Chem. 1963;27:738–739.10.1271/bbb1961.27.738
- de Mayo P, Spencer EY, White RW. The constitution of helminthosporal. J Am Chem Soc. 1962;84:494–495.10.1021/ja00862a037
- White GA, Taniguchi E. The mode of action of helminthospal. II. Effect on the permeability of plant cell membranes. Can J Bot. 1972;50:1415–1420.10.1139/b72-170
- Carlson H, Nilsson P, Jansson HB, et al. Characterization and determination of prehelminthosporol, a toxin from the plant pathogenic fungus Bipolaris sorokiniana, using liquid chromatography/mass spectrometry. J Microbial Meth. 1991;13:259–269.10.1016/0167-7012(91)90063-V
- Nilsson P, Åkesson H, Jansson HB, et al. Production and release of the phytotoxin prehelminthosporol by Bipolaris sorokiniana during growth. FEMS Microb Ecol. 1993;102:91–98.10.1111/fml.1993.102.issue-2
- Briquet M, Vilret D, Goblet P, et al. Plant cell membranes as biochemical targets of the phytotoxin helminthosporol. J Bioenerg Biomembr. 1998;30:285–295.10.1023/A:1020553005293
- Nakajima H, Isomi K. Hamasaki T and Ichinoe M. Sorokinianin: a novel phytotoxin produced by the phytopathogenic fungus Bipolaris sorokiniana. Tetrahedron Lett. 1994;35:9597–9600.10.1016/0040-4039(94)88520-6
- Tamura S, Sakurai A. Syntheses of several compounds related to helminthosporol and their plant growth-regulating activities. Agric Biol Chem. 1964;28:337–338.10.1080/00021369.1964.10858247
- Tamura S, Sakurai A, Kainuma K, et al. Syntheses of several compounds related to helminthosporol and their plant-growth regulating activities. Agric Biol Chem. 1965;29:216–221.10.1080/00021369.1965.10858370
- Hashimoto T, Sakurai A, Tamura S. Physiological activities of helminthosporol and helminthosporic acid: I. Effects on growth of intact plants. Plant Cell Physiol. 1967;8:23–34.10.1093/oxfordjournals.pcp.a079248
- Hashimoto T, Tamura S. Physiological activities of helminthosporol and helminthosporic acid: III Effects on seed germination. Plant Cell Physiol. 1967;8:197–200.10.1093/oxfordjournals.pcp.a079241
- Kamiya Y, Kobayashi M, Fujioka S, Yamane H. Nakayama I and Sakurai A. Effects of a plant growth regulator, prohexadione calcium (BX-112), on the elongation of rice shoots caused by exogenously applied gibberellins and helminthosporol, part II. Plant Cell Physiol. 1991;32:1205–1210.
- Yabuta T, Sumiki Y. On the crystal of gibberellin, a substance to promote plant growth. J Agric Chem Soc Jpn. 1938;14:1526.
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005;437:693–698.10.1038/nature04028
- Shimada A, Ueguchi-Tanaka M, Nakatsu T, et al. Structural basis for gibberellin recognition by its receptor GID1. Nature 2008;456:520–523.10.1038/nature07546
- Sun TP. Gibberellin-GID1-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiol. 2010;154:567–570.10.1104/pp.110.161554
- Griffiths J, Murase K, Rieu I, et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 2006;18:3399–3414.10.1105/tpc.106.047415
- Nakajima M, Shimada A, Takashi Y, et al. Identification and characterization of Arabidopsis gibberellin receptors. Plant J. 2006;46:880–889.10.1111/tpj.2006.46.issue-5
- Jiang K, Otani M, Shimotakahara H, et al. Substituted phthalimide AC94377 is a selective agonist of the gibberellin receptor GID1. Plant Physiol. 2017;173:825–835.10.1104/pp.16.00937
- Peng J, Carol P, Richards DE, et al. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11:3194–3205.10.1101/gad.11.23.3194
- Silverstone AL, Jung H-S, Dill A, et al. Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell. 2001;13:1555–1566.10.1105/tpc.13.7.1555
- Phillips AL, Ward DA, Uknes S, et al. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995;108:1049–1057.10.1104/pp.108.3.1049
- Mimura M, Nagato Y, Itoh J. Rice PLASTOCHRON genes regulate leaf maturation downstream of the gibberellin signal transduction pathway. Planta 2012;235:1081–1089.10.1007/s00425-012-1639-5
- Ogawa M, Hanada A, Yamauchi Y, et al. Gibberellin biosynthesis and response during arabidopsis seed germination. Plant Cell. 2003;15:1591–1604.10.1105/tpc.011650
- Yoon JM, Nakajima M, Mashiguchi K, et al. Chemical screening of an inhibitor for gibberellin receptors based on a yeast two-hybrid system. Bioorg Med Chem Lett. 2013;23:1096–1098.10.1016/j.bmcl.2012.12.007
- Coombe BG, Mander LN, Paleg LG, et al. Gibberellin-like activity of helminthosporic acid analogues. Aust J Plant Physiol. 1974;1:473–481.10.1071/PP9740473
- Dassault Systèmes BIOVIA. Discovery studio modeling environment, release 2017. San Diego, CA: Dassault Systèmes; 2015.