Abstract
An enzymatic method for 6-oxohexanoic acid production was developed using 6-aminohexanoic acid and an ω-amino group-oxidizing enzyme (ω-AOX) from Phialemonium sp. AIU 274. 6-Oxohexanoic acid was produced from 6-aminohexanoic acid with 100% yield by incubation with 0.3 U of the ω-AOX and 20 U of catalase at 30 °C for 30 h in 0.1 M potassium phosphate buffer (pH 7.0).
Poly(ε-caprolactone) (PCL) is a useful material for scaffolds in tissue engineering and micellar drug carriers for drug delivery.Citation1–3) PCLs that have functional side groups such as hydrophilic moieties, halogens, amines, and unsaturated functional groups have also attracted a lot of attention. These substituted PCLs exhibit tunable crystallinity, hydrophilicity, biodegradation, bioadhesion, and mechanical properties.Citation4–6) 6-Oxohexanoic acid is a promising candidate as a raw material for the synthesis of functionalized PCLs with tunable properties.Citation7) However, the starting material for the chemical synthesis of functional PCLs such as 6-oxohexanoic is very expensive, and the chemical synthesis of 6-oxohexanoic acid from 2-hydroxycyclohexanone dimer utilizes hazardous compounds.Citation8) For example, tetrahydrofuran is used as a solvent and sodium periodate is added as an oxidizing agent. Thus, new methods for synthesis of 6-oxohexanoic acid from inexpensive compounds using safe catalysts are required. In this study, we developed an enzymatic method for producing 6-oxohexanoic acid from 6-aminohexanoic acid, which is easily produced by ring opening of caprolactamCitation9) and is much cheaper than 2-hydroxycyclohexanone dimer.
Recently, we isolated a fungal strain, Phialemonium sp. AIU 274, as a producer of a new enzyme (ω-AOX) exhibiting oxidase activity for a wide variety of ω-amino compounds, such as ω-aminocarboxylic acids, ω-aminoalcohols, monoamines, and diamines.Citation10) The ω-AOX efficiently catalyzes oxidative deamination of long- and medium-chain substrates, resulting that 6-aminohexanoic acid was oxidized to 6-oxohexanoic acid (Fig. ).Citation10) These findings strongly suggested that the ω-AOX enable to produce 6-oxohexanoic acid from 6-aminohexanoic acid. Therefore, we optimized the reaction conditions of ω-AOX from Phialemonium sp. AIU 274 to develop a new enzymatic method for the production of 6-oxohexanoic acid.
Fig. 1. A reaction scheme of oxidation of 6-aminohexanoic acid into 6-oxohexanoic acid by the ω-amino group oxidizing enzyme from Phialemonium sp. AIU 274 (ω-AOX).
Phialemonium sp. AIU 274 strain was cultivated in a 6-aminohexanoic acid medium (0.3% 6-aminohexanoic acid, 0.5% glucose, 0.2% KH2PO4, 0.1% Na2HPO4, and 0.05% MgSO4·7H2O, pH 7.0) according to our previous report.Citation10) The ω-AOX was purified according to our previous report, using an ammonium sulfate fractionation and four column chromatographies of Phenyl-Toyopearl 650 M, DEAE-Toyopearl 650 M, hydroxyapatite, and Toyopearl HW-5.Citation10) The ω-AOX activity was spectrophotometrically assayed at 30 °C by measuring the reaction velocity of hydrogen peroxide formation at 555 nm of absorbance, using 20 μmol of 6-aminohexanoic acid at pH 7.0 (reaction volume, 1 mL).Citation10) One unit of enzyme activity was defined as the amount of enzyme catalyzing the formation of one micromole of hydrogen peroxide per minute.
First, we analyzed the effects of pH on enzyme activity and stability using the purified ω-AOX (1.03 U/mg). In assays between pH 5.0 and pH 8.5, the maximal oxidase activity toward 6-aminohexanoic acid was obtained at pH 7.5 (Fig. (A)). Then, the effect of pH on enzyme stability was determined by incubation between pH 5.0 and pH 8.5 and 30 °C for 30 min without substrate. The ω-AOX was stable between pH 6.5 and 7.5 (more than 70% of the enzyme activity remained) (Fig. (A)). These results indicated that a long time-reaction for 6-aminohexanoic acid oxidation to produce 6-oxohexanoic acid is optimal at pH 7.0.
Fig. 2. Effects of pH and temperature on the activity and stability of the ω-AOX. (A) The reaction rate for 6-aminohexanoic acid oxidation was assayed under standard conditions except pH (closed circles). The pH stability was assayed under standard conditions after heating at 30 °C or 30 min at the indicated pH (open circles). The percentage of remaining activity was obtained as a ratio to that without heating. (B) The reaction rate for 6-aminohexanoic acid oxidation was assayed under standard conditions except for reaction temperature (closed circles). Thermal stability was assayed under standard assay conditions after the enzyme solution was incubated at pH 7.0 for 30 min at the indicated temperatures (open circles). The percentage of remaining activity was obtained by the ratio to activity without heating.
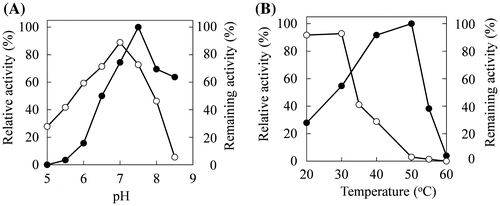
When the reaction temperature for 6-aminohexanoic acid oxidation of the ω-AOX was analyzed between 20 and 60 °C at pH 7.0, the highest activity was obtained at 50 °C and decreased remarkably above 55 °C (Fig. (B)). The effect of temperature on enzyme stability was also analyzed by incubation at pH 7.0 for 30 min without substrate. The ω-AOX was stable below 30 °C at pH 7.0, but not above 35 °C (Fig. (B)). Thus, it was revealed that the initial reaction velocity was fastest at 50 °C but this speed was not maintained for a long time at 50 °C.
Therefore, we analyzed the optimal temperature for 6-oxohexanoic acid production by determining the reaction product, 6-oxohexanoic acid, as follows. First, 200 mM 6-aminohexanoid acid was incubated with 0.2 U of the ω-AOX in 0.1 M potassium phosphate buffer, pH 7.0, at different temperatures from 10 to 40 °C for 48 h with shaking (120 strokes/min). The reaction was terminated by incubation at 100 °C for 3 min, and the resulting precipitates were removed by filtration using Vivaspin 6 (Sartorius, Göttingen, Germany). 6-Oxohexanoic acid in the supernatant was measured by high performance liquid chromatography (HPLC) with a TSKgel DEAE-5PW column (Tosoh, Tokyo, Japan). Separations were performed using a programmed gradient of solvent A (water) and solvent B (water with 0.3 M NaCl) (0% B, 0–5 min: 100% B for 5–15 min: 100% B 15–25 min) with a flow rate of 0.8 ml/min at 40 °C. Detection was monitored at 210 nm. Among the reaction temperatures mentioned above, the maximum production of 6-oxohexanoic acid was obtained at 30 °C (Fig. (A)). Thus, we concluded that the optimal pH and temperature for 6-oxohexanoic acid production were pH 7.0 and 30 °C, respectively.
Fig. 3. Analysis for optimizing the reaction conditions of 6-oxohexanoic acid production. (A) Effect of temperature on 6-oxohexanoic acid production. Two hundred millimolar of 6-aminohexanoic acid was incubated with 0.2 U of the ω-AOX at 10 °C – 40 °C for 48 h in 0.1 M potassium phosphate buffer (pH 7.0). (B) Effect of the ω-AOX amounts on 6-oxohexanoic acid production. Two hundred millimolar of 6-aminohexanoic acid was incubated with indicated amounts of ω-AOX at 30 °C for 6, 18, 36, and 48 h, in 0.1 M potassium phosphate buffer (pH 7.0). Hatched bars, 6 h; gray bars, 18 h, open bars, 36 h, closed bars, 48 h. (C) Effect of catalase addition on 6-oxohexanoic acid production. Two hundred millimolar of 6-aminohexanoic acid was incubated with 0.3 U of the ω-AOX at 30 °C for 48 h in 0.1 M potassium phosphate buffer (pH 7.0) (Open circles). The same reaction was carried out by adding 20 U of catalase in the above reaction mixture (Close circles). (D) Effect of H2O2 on the stability of the ω-AOX. Two hundred milliunit of the ω-AOX was incubated with H2O2 at 30 °C for 1 h in 0.1 M potassium phosphate buffer (pH 7.0), and 2 mU of ω-AOX was used for enzyme assay. The ω-AOX activity was assayed at 30 °C by measuring the hydrogen peroxide formation at 505 nm of absorbance, using the reaction solution 20 μmol of 6-aminohexanoic acid, 0.74 μmol of 4-aminoantipyrine, 0.034 μmol of phenol and 15 units of peroxidase at pH 7.0. The percentage of remaining activity was obtained by the ratio to activity without H2O2 and heating. The data represent the means ± S.D. (n = 3).
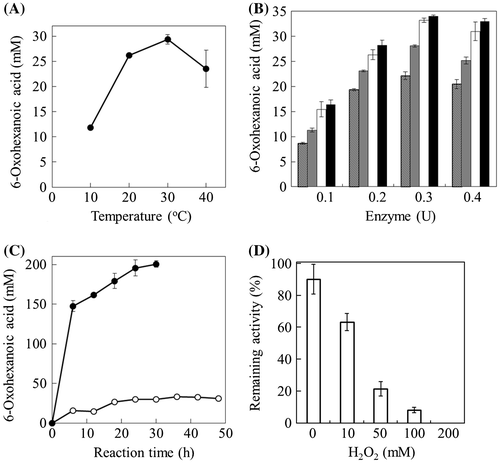
Next, the optimal amounts of enzyme and reaction time were examined under the optimized pH and temperature conditions. When the enzyme amounts were varied between 0.1 and 0.4 U, the product amount of 6-oxohexanoic acid increased along with the enzyme amounts up to 0.3 U (Fig. (B)). In the case of 0.3 U of ω-AOX, the amount of 6-oxohexanoic acid increased until 36 h of incubation but did not increase by prolonging the incubation time (Fig. (C)). Thus, the highest production of 6-oxohexanoic acid was achieved by incubation with 0.3 U of ω-AOX for 36 h. However, the yield of 6-oxohexanoic acid was approximately 33 mM (yield was 17%). Since we had demonstrated that hydrogen peroxide generated by alcohol oxidase reaction affects the conversion yields of glyoxal from ethylene glycol,Citation11) we investigated the effect of H2O2 on the enzyme stability by incubation at pH 7.0 for 1 h without substrate. Although more than 90% of activity remained in the absence of H2O2, remaining activity decreased along with the H2O2 concentration (from 10 to 200 mM) (Fig. (D)). The ω-AOX was completely deactivated by 200 mM H2O2.
Therefore, we added catalase into the reaction mixture for 6-oxohexanoic acid production to eliminate hydrogen peroxide generated by ω-AOX reaction. When 6-oxohexanoic acid production was performed under the optimal conditions by adding 20 U of catalase, the 6-oxohexanoic acid production significantly increased (Fig. (C)). Furthermore, 200 mM 6-aminohexanoic acid substrate was completely converted into 6-oxohexanoic acid by incubation with 0.3 U of ω-AOX and 20 U of catalase at 30 °C and pH 7.0 for 30 h. These results indicated that hydrogen peroxide generated by oxidase reaction affects the stability of the oxidase.
In conclusion, we first developed a new enzymatic method for producing 6-oxohexanoic acid with a 100% conversion yield, versus the 80% yield of the chemical method, and with a low raw material cost.Citation8) Since our enzymatic method is attractive, we will further study the method to combine with PCL synthesis in our future work. For the further improvement, we are now conducting gene cloning from genomic DNA of Phialemonium sp. AIU 274 as well as overexpression of the ω-AOX in different hosts to increase the enzyme productivity.
Author contribution
Miwa Yamada and Kimiyasu Isobe designed the experiments. Miwa Yamada, Mitsuaki Ooe, and Tomoko Sasaki performed the experiments. Masao Miyazaki contributed to data analyses and discussion. Miwa Yamada wrote the manuscript.
Funding
This work was partially supported by a Grant-in-Aid for Scientific Research of Japan [grant number 258203950001] (to M. Yamada) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank Dr Hitoshi Shimoi from Iwate University for valuable discussions.
References
- Rainbolt E, Washington K, Biewer M, et al. Recent developments in micellar drug carriers featuring substituted poly(ε-caprolactone)s. Polym Chem. 2015;6:2369–2381.10.1039/C4PY01628A
- Place ES, George JH, Williams CK, et al. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev. 2009;38:1139–1151.10.1039/b811392 k
- Woodruff M, Hutmacher D. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog Polym Sci. 2010;35:1217–1256.10.1016/j.progpolymsci.2010.04.002
- Hao J, Rainbolt E, Washington K, et al. Synthesis of functionalized poly(caprolactone)s and their application as micellar drug delivery systems. Curr Org Chem. 2013;17:930–942.10.2174/1385272811317090007
- Labet M, Thielemans W. Synthesis of polycaprolactone: a review. Chem Soc Rev. 2009;38:3484–3504.10.1039/b820162p
- Seyednejad H, Ghassemi AH, van Nostrum CF, et al. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J Control Release. 2011;152:168–176.10.1016/j.jconrel.2010.12.016
- Zhang J, Zhang M, Du F-S, et al. Synthesis of functional polycaprolactones via passerini multicomponent polymerization of 6-oxohexanoic acid and isocyanides. Macromolecules. 2016;49:2592–2600.10.1021/acs.macromol.6b00096
- Bouet A, Oudeyer S, Dupas G, et al. New advances in stereoselective Meyers’ lactamization. Application to the diastereoselective synthesis of β-substituted oxazoloazepinones. Tetrahed: Asym. 2008;19:2396–2401.
- O’Neil MJ, Heckelman PE, Koch CB, et al. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. 14th ed. Whitehouse Station, NJ: Merck Research Laboratories, division of Merck & Co.; 2006.
- Isobe K, Sasaki T, Aigami Y, et al. Characterization of a new enzyme oxidizing ω-amino group of aminocarboxyric acid, aminoalcohols and amines from Phialemonium sp. AIU 274. J Mol Cat B: Enzym. 2013;96:89–95.10.1016/j.molcatb.2013.06.012
- Isobe K, Nishise H. Enzymatic production of glyoxal from ethylene glycol using alcohol oxidase from methanol yeast. Biosci Biotechnol Biochem. 1994;58:170–173.10.1271/bbb.58.170
