Abstract
Thermophilic ammonium-tolerant bacterium Bacillus sp. TAT105 grows and reduces ammonia (NH3) emissions by assimilating ammonium nitrogen during composting of swine feces. To evaluate the efficacy of a biological additive containing TAT105 at reducing NH3 emissions, composting tests of swine manure on a pilot scale (1.8 m3) were conducted. In the TAT105-added treatment, NH3 emissions and nitrogen loss were lower than those in the control treatment without TAT105. No significant difference was detected in losses in the weight and volatile solids between the treatments. Concentration of thermophilic ammonium-tolerant bacteria in the compost increased in both treatments at the initial stage of composting. In the TAT105-added treatment, bacterial concentration reached ~109 colony-forming units per gram of dry matter, several-fold higher than that in the control and stayed at the same level until the end. These results suggest that TAT105 grows during composting and reduces NH3 emissions in TAT105-added treatment.

Composting is a treatment method for producing compost from organic wastes for use as plant fertilizer or for soil amendment. During this treatment, organic matter in the waste, including phytotoxic substrates is decomposed and stabilized by microorganisms. In parallel with these processes, drying of the material and killing of pathogens and contaminating weed seeds are promoted by the heat generated by organic matter decomposition.Citation1–3) Composting is widely used for treatment and recycling of livestock wastes.Citation4–6) During the composting of livestock wastes, however, a large amount of environmentally harmful gaseous substances, such as malodorous compounds and greenhouse gasses, are emittedCitation7–9); this issue is considered one of the environmental problems caused by livestock production. When organic matter in the wastes is actively decomposed and temperature of the material rises substantially (to 60–80 °C), concentrated malodorous compounds are emitted. In particular, ammonia (NH3) is released at quite high concentrations for a long period, and therefore, NH3 is regarded as the main component of the malodor from composting.Citation7) It causes not only malodor complaints from the neighborhood community around the treatment facilities but also global environmental pollution, such as acid rain and soil acidification.Citation10) Additionally, nitrogen loss via NH3 emissions decreases the value of the compost as fertilizer. Therefore, achieving a reduction in NH3 emissions is an important task in the composting of livestock wastes.
In the composting process, decomposition of organic matter and heat generation accompanying it result in high temperature and concentrated NH3 in the piled livestock wastes, as described above. If a microorganism that adapts to such environmental conditions and assimilates ammonium nitrogen (-N) effectively is added to the livestock wastes, it could grow advantageously and reduce NH3 emissions during the composting. In our previous studies, a thermophilic ammonium-tolerant bacterium Bacillus sp. TAT105, was isolated and found to grow during the composting treatment of swine feces, while assimilating
-N; the reduction in NH3 emissions by the addition of TAT105 was evaluated in laboratory-scale composting tests of swine feces.Citation11,12) When TAT105 was added to the material so that the final concentration of TAT105 in the material was above 107 cells/(g of dry matter [gDM]) at the start of composting, NH3 emissions and nitrogen loss during the composting tended to be lower in the TAT105-added treatment than in the control treatment (without TAT105).Citation11,12) It was also confirmed that the dried solid culture of TAT105 could be stored for a long period without a significant decrease in the concentration of bacteria, and NH3 emissions were reduced by adding the dried solid culture to the composting mass, suggesting that TAT105 can serve as a biological additive.
Additionally, an attempt was made to evaluate the growth of thermophilic ammonium-tolerant bacteria (TAT), including TAT105, during the composting treatment by a colony formation assay on an agar medium containing 1 M of NH4Cl at 60 °C.Citation12) All the colonies emerging on this agar medium were morphologically identical to those of TAT105, and these strains had a unique profile of restriction fragment length polymorphism of the 16S rRNA gene digested with HinfI or HaeШ: identical to the profile of TAT105 and different from those of the species phylogenetically close to TAT105.In the composting tests, TAT concentrations in the composted materials evaluated by this method increased remarkably in both the TAT105-added and control treatments by the end of composting, and the concentration of TAT reached a higher level in TAT105-added treatment than in the control.Citation11,12) These results suggested that these culture conditions have high selectivity for TAT105 or its close relatives and can be used for comparison of TAT concentrations between TAT105-added and control treatments during composting.
The efficacy of addition of TAT105 at reducing NH3 emissions depends on the growth of TAT105 in the material during the composting. The NH3 emissions and growth of microorganisms should be affected by the scale of treatment and characteristics of the materials. To confirm the applicability of this method, the efficacy of adding TAT105 should be evaluated during composting of the materials on a scale closer to that of practical composting treatments. In this study, composting tests of swine manure discharged from a pig farm were carried out on a pilot scale (weight 800–1,000 kg and volume 1.8 m3) using a biological additive containing TAT105, to evaluate its efficacy at reducing NH3 emissions at the scale and management close to those in practical composting treatments.
Materials and methods
Preparation of the biological additive
The biological additive containing TAT105 (the TAT105 additive), dried solid culture of TAT105, was prepared as described in our previous study.Citation12) TAT105 was cultured in a liquid medium (YA medium) containing 0.5% of yeast extract (Difco Laboratories Inc., Detroit, MI), 100 mM NH4Cl, and several organic and inorganic salts, by shaking at 50 °C for 16 h, and this seed culture was inoculated into a solid medium containing wheat bran, a liquid medium made of the same materials as in the YA medium, 10 μM MnCl2·4H2O, and 1.5% soluble starch, and was incubated at 55 °C for 4 days. Among the materials of the solid medium, coaled pulp sludge was replaced by activated granular charcoal (Wako Pure Chemical Industries Ltd., Osaka, Japan) ground with a mortar and pestle. After the harvesting, the culture was dried at 45 °C for 3 days. The preparation was subdivided into several periods, and the additive was kept in vinyl bags at room temperature (10–30 °C) until use.
The composting facility
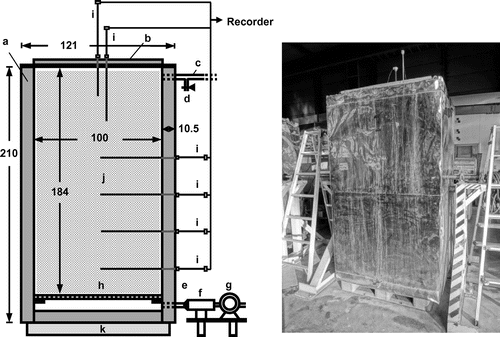
Figure shows the composting facility used in this study. The main part is a rectangular parallelepiped tank (121 cm length and width, 210 cm height, capacity ≈ 1.8 m3) which consists of the outer shell of a stainless-steel panel and the inside filling of insulation material (polystyrene board). Air supplying and air exhaust pipes are located near the bottom and top of the back wall, respectively. After addition of the material to be composted, a cover (made of the same materials as the tank is) was placed on top of the tank and fastened with bolts. An aeration pump and an air heater are connected to the air-supplying pipe, and continuous aeration with air warmed to 30 °C is supplied during the composting treatment. The thermocouple sensor rods were inserted at the height of 20, 50, 80, 110, 140, and 160 cm of the mixture, through the back wall of the tank and the cover, and the material temperatures were recorded during the composting treatment.
Fig. 1. The scheme of the composting facility.
Management of the composting test
Table shows the settings of the composting test. Swine manure were collected on a pig farm in the Kamoto district, and sawdust was purchased from Koshi Bio-X, a composting center in Koshi city, both in Kumamoto Prefecture, at the respective periods of the composting tests. One thousand kilograms of swine manure and 200 kg of sawdust (weight ratio 5:1) were mixed using a manure spreader (DH1600, Takakita Co., Ltd., Nabari, Japan). One day before the start of composting, 3 kg of the TAT105 additive and 3 L of water were mixed and kept at room temperature, and this mixture was mixed with the manure and sawdust immediately before the start of composting (TAT-added). The dose and pre-treatment with the TAT105 additive were based on the results of the laboratory-scale composting tests in our previous study.Citation12) In the control treatment, a mixture of the same weights of manure and sawdust without the additive was prepared (Control). Those mixtures were placed in the tanks at the composting facilities. The mixtures were composted for 4 weeks under continuous aeration. To avoid the influence of ambient temperature on the progress of composting, the inlet air warmed to 30 °C by an air heater was supplied. The air flow rate was changed every week: 120, 100, 80, and 60 L/min in the first, second, third, and fourth week. During the composting, the mixtures were removed from the tanks, completely mixed and placed in the tanks again (turning), at an interval of 1 week. At the start and at the end, and before and after every turning, the mixtures were weighed, and after the turning, 3 kg of mixtures were collected from both treatments for analyses. At the second turning (2 weeks after the start), 150 L of water was added to the mixture to avoid stagnation of organic matter decomposition because of excess drying of the mixture.
Table 1. Setting of the composting test.
During the composting test, NH3 concentration in the exhaust gas from the tank was monitored at 12- or 24-h intervals. At every monitoring time point, the exhaust gas was collected from the gas sampling port (part “d” in Fig. ) via a flask (500-mL volume) connected to the port with a polytetrafluoroethylene tube, for cooling of the gas and removal of moisture, and the NH3 concentration was determined using an NH3 detection tube (No. 3L, 3M, and 3HM, GASTEC Co., Ayase, Japan).
The composting test with the same settings was repeated three times during 2013–2015.
Analyses of mixtures
The collected mixtures in the composting tests were subjected to the analyses similar to those in the laboratory-scale composting tests in our previous studies,Citation11,12) except for the volumes, several handling procedures, and the analytical method described below. The analyses were conducted in duplicate, except for some items the number of repetitions was noted. Finally, the average values of three composting tests were calculated.
Approximately 170 g of a sample was placed in an evaporating dish (capacity of 400 mL) and dried at 105 °C for 2 days, and subsequently incinerated at 550 °C for 6 h. The dish with the mixture was weighed before and after the respective incubations, and the moisture content (MC) and volatile solids (VS), roughly considered as organic matter content, were calculated.
Kjeldahl nitrogen (Kj-N) was analyzed by the method of BremnerCitation13). Approximately 5 g of a sample was put into a digestion tube (capacity 350 mL), and 15 mL of H2SO4, 20 mL of distilled water, and the Kjeldahl catalyst (Kjeltabs, Thompson & Capper Ltd., UK) were added. The tube was set on a heater (Foss Tecator DS-20, Foss Japan Ltd., Tokyo) and incubated at 420 °C for ~3 h. After cooling, 100 mL of distilled water was added to the decomposed sample, and then subjected to distillation analysis using an automatic analyzer (Super Kjel 1200, Actac Co., Ltd., Tokyo). The analysis was carried out six times per sample. The total amount of nitrous and nitric nitrogen (NOx-N) was also analyzed by the method of Bremner and KeeneyCitation14) as described in our previous study.Citation10) The concentrations of these nitrogen contents were calculated as the percentages of dry matter of the sample (%DM), and total nitrogen (TN) in each sample was calculated as the sum of Kj-N and NOx-N. On the basis of these analyses and the weights of the mixtures, the losses and remainders of VS and TN of the mixtures were calculated.
The concentrations of TAT in the TAT105 additive and in the composted mixtures in the composting tests were determined by colony formation on an agar medium containing yeast extract (Difco Laboratories Inc.), 1 M NH4Cl, several salts, and 3.5% of agar (YA1 agar) as described elsewhere.Citation12) Thirty grams of a sample was homogenized with 270 mL of a physiological salt solution (PSS, 0.85% NaCl) in a 500-mL cup at 18,000 rpm with a blender (AM-5, Nihon Seiki Kaisha Ltd., Tokyo). This suspension was subjected to serial dilution with PSS and then was inoculated into YA1 agar. After incubation at 60 °C for 2 days, the emerging colonies were counted, and colony-forming units per gram of dry matter of the sample (CFU/gDM) were calculated. The concentration of TAT105 in the TAT105 additive determined by this method was 3.5 × 109 CFU/gDM on average.
The results of analyses in both treatments were subjected to Student’s t-test. In the analysis of TAT concentrations in the mixtures, the logarithms of the values were used. In Results and Discussion, the average values calculated from repeated composting tests are shown.
Results
Changes in the temperatures of the mixtures, NH3 emissions, and the characteristics of the mixtures in the composting tests
Figure shows changes in the temperatures of the mixtures and NH3 concentrations in the exhaust gasses during the composting tests. In both treatments, temperatures were higher at the height of 140 cm during almost all the composting periods. The temperatures rose above 70 °C in ~3 days from the start, and a rapid rise followed by a gradual decline was observed after every turning. The peak values on average were 74.4 and 75.2 °C in Control and TAT-added, respectively, as recorded at ~9.5 days from the start. Ammonia concentrations in the exhaust gasses gradually increased to 1,000–2,000 ppm after 3–3.5 days from the start, and repeated the remarkable rise and fall after the first and second turning. During days 4–7, 8–11, and 15.5–16.5 from the start, NH3 concentrations were >1,000 ppm, and in those periods, the concentrations in TAT-added tended to be lower than those in Control. The peak values on average were 5,170 ppm in Control and 3,730 ppm in TAT-added, detected at 8.5 days from the start. After the third turning, the concentrations remained within 1,000 ppm in both treatments until the end.
Fig. 2. Changes in temperatures of the mixtures and NH3 concentrations in exhaust gasses during the composting tests.
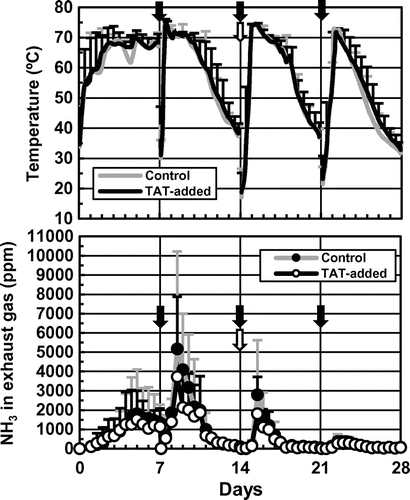
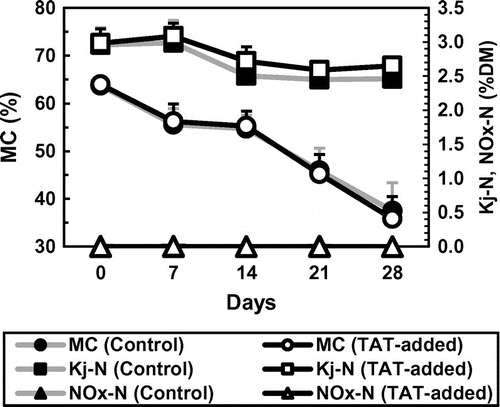
Figure shows changes in MCs and nitrogen contents in the mixtures during the composting tests. The initial MCs were 63.73 and 63.97% in Control and TAT-added, respectively, and decreased to 37.48 and 35.83% until the end. The initial Kj-N concentrations were 2.96%DM and 2.98%DM in Control and TAT-added, respectively, and they decreased to 2.46 and 2.65% until the end. On the contrary, NOx-N levels were barely detectable (<0.01%DM) and TN concentrations were nearly equal to Kj-N concentrations in both treatments throughout all the composting tests. It has been pointed out that, during composting of livestock manure, accumulation of NOx-N gradually takes place in the composted manure after active decomposition of organic matter with heat generation, and substantial NH3 emission abates.Citation3,4) This observation suggested that nitrification of ammonium nitrogen did not proceed in the composted mixtures during the composting tests here. In these parameters, significant differences between Control and TAT-added were not detected throughout the composting period; however, Kj-N concentrations tended to be a little higher in TAT-added than in Control after the first turning.
Changes in weights, VS, TN, and TAT concentrations in the mixtures in the composting tests
The weights, VS contents, and TN contents of the mixtures in the composting tests changed as shown in Fig. . The total amounts of these substances in the initial mixtures were not significantly different between the two treatments. The average values of the initial weights were 912.2 and 917.2 kg in Control and TAT-added, respectively, and considering the 150 L of water added at the second turning, total applied weights were 1,62.2 and 1,067.2 kg. At the end of composting, the weights of the remainders were 376.8 kg in Control and 374.7 kg in TAT-added (Fig. (a)). The ratios of the remainder to the total weight of applied materials were 35.5% in Control and 35.1% in TAT-added. Total VS amounts in the initial mixtures were 301.7 and 300.4 kg in Control and TAT-added, respectively, and decreased to 206.6 and 211.3 kg by the end of composting, corresponding to 68.5% and 70.4% of the initial values (Fig. (b)). The remainders and losses of the weights and VS contents were not significantly different between TAT-added and Control throughout the composting tests, suggesting that decomposition of organic matter progressed to a similar extent in both treatments.
Fig. 4. Changes in weights, VS contents, and TN contents in the mixtures during the composting tests.
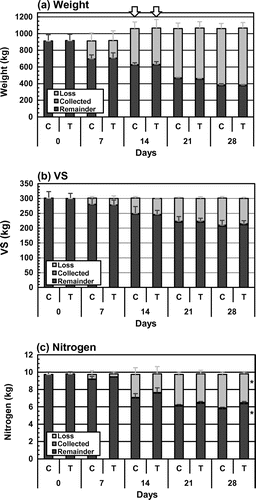
TN amounts in the initial mixtures were 9.75 and 9.82 kg in Control and TAT-added, respectively, and decreased to 5.77 and 6.36 kg by the end, corresponding to 59.2 and 64.8% of the initial values (Fig. (c)). The nitrogen losses during the composting, except for the amounts in the samples collected at the turnings, were 3.86 kg in Control and 3.33 kg in TAT-added, corresponding to 39.6 and 33.9% of the respective TN in the initial mixtures, and 14.4% (±2.9% [SD]) lower in TAT-added than in Control. Significant differences (p < 0.05) were observed in the loss during the composting and in the remainder of the final materials between Control and TAT-added.
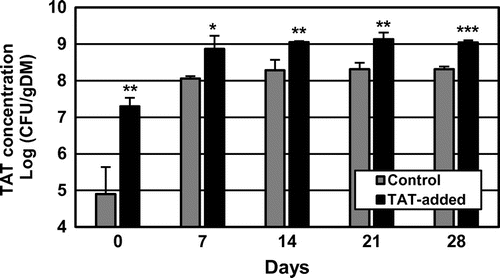
Figure shows the changes in the concentrations of TAT in the composted mixtures. The concentrations at the start were 1.7 × 105 and 2.2 × 107 CFU/gDM in Control and TAT-added, respectively. They increased remarkably until the first turning (7 days after the start), and the concentration reached 9.5 × 108 CFU/gDM in TAT-added: ~eight-fold higher than that in Control (1.2 × 108 CFU/gDM). Thereafter, they changed little in both treatments until the end of composting, and the concentrations in TAT-added consistently stayed at ~109 CFU/gDM (five–eight-fold higher than in Control), and significant differences (p < 0.001–0.05) were observed in the logarithmic values of the concentrations between the treatments at each turning and at the end.
Fig. 5. Changes in TAT concentrations in the mixtures during the composting tests.
Discussion
Adding some microorganisms to livestock feed or livestock wastes to reduce odor emissions is regarded as a popular approach, and many biological additives on the market are advertised as reducing odor emission. In many cases, however, the microorganisms in the additives are not known exactly, and the effects are ambiguous and unstable.Citation15,16)
The present study was carried out as part of a trial to utilize a microorganism for reducing NH3 emissions during composting of livestock wastes, including identification of the microorganism, evaluation of the efficiency, and development of a utilization method.Citation11,12)
The lower NH3 concentrations in the exhaust gasses during the composting (Fig. ), the smaller nitrogen loss, and the larger nitrogen remainder were suggestive of smaller NH3 emissions during the composting in TAT105-added than in Control (Fig. (c)). On the contrary, a significant difference was not detected in the loss and remainder of VS between the two treatments (Fig. (b)), indicating that decomposition of organic matter progressed similarly in both treatments, and that the smaller nitrogen loss in TAT-added was not caused by stagnation of decomposition. Additionally, it seemed that nitrification neither occurred nor affected NH3 emissions in both treatments during the composting tests (Fig. ). These results were similar to the findings of the laboratory-scale tests.Citation11,12)
In TAT-added treatment, the concentration of TAT in the mixture increased more than it did in Control in the first 7 days of composting, and afterward, it stayed at the similar level until the end (Fig. ), indicating that nitrogen amount assimilated and kept by TAT as biomass was larger in TAT-added than in Control. Usually, thermophilic microorganisms, including TAT, are present at a low concentration (<106 CFU/gDM) in swine feces.Citation11) Besides, in this study, TAT were present in the mixtures in Control at a concentration less than 5 × 105 CFU/gDM at the start of composting. They were assumed to be TAT105 or its close relatives,Citation12) and they might grow assimilating -N. Nevertheless, the increase of TAT in Control during composting was smaller than that in TAT-added. On the contrary, in TAT-added, the concentration of TAT105 in the mixture at the start was forced to be above 107 CFU/gDM by addition of the TAT105 additive, according to the effective dose determined in our previous study,Citation12) and TAT concentration increased and became several-fold greater than that in Control. These observations suggested that TAT105 became the dominant strain among the thermophilic microorganisms in the mixtures in TAT-added at the start, and this situation led to advantageous growth of TAT105 in the initial stage of composting at a high temperature and high
-N concentration. This larger increase in TAT in TAT105-added treatment was observed in the composting tests with high reproducibility, as in the laboratory-scale composting tests in our previous studies,Citation11,12) and is likely to be the mechanism underlying the reduction in NH3 emissions.
Compared with the results of the laboratory-scale composting tests in our previous study,Citation12) the effectiveness in reducing NH3 emissions was lower in the pilot-scale composting. The TAT concentration in TAT-added at the end of the pilot-scale test was 1.1 × 109 CFU/gDM: 1/3 of the value in the laboratory-scale test (3.4 × 109 CFU/gDM). The swine manure used in this study had higher humidity and nitrogen content than the swine feces used in the laboratory-scale tests (data not shown), pointing to contamination with a certain amount of urine. Additionally, a larger amount of sawdust was added to the swine manure, for control over humidity of the mixtures of materials at the start of composting; the weight ratios (sawdust/feces or sawdust/manure) were 1/6 and 1/5 in laboratory scale and pilot scale tests, respectively. For these reasons, it is likely that the concentrations of usable carbon sources for TAT105 were lower while the concentrations of nitrogen were higher in the mixtures in the pilot-scale tests, thereby causing smaller growth of TAT105, larger NH3 emissions, and consequently, lower efficacy of the addition of TAT105.
The results of this study indicate that the effective dose and pretreatment settings for the use of TAT105 that were determined in the laboratory-scale composting testsCitation12) are suitable for the pilot-scale composting and may be applied to practical composting treatment. Discharged volume and characteristics of the manure are different among farms, and composting treatments of manure vary widely in scale, the type of facility, and management during the treatment and treatment period.Citation3,5,17) These factors should affect NH3 emissions during the composting, and therefore, the efficacy of TAT105 at reducing NH3 emissions; the nitrogen loss should vary depending on these parameters. During application of TAT105 to the practical composting treatments, the efficacy and optimal conditions for TAT105 should be carefully evaluated. In a future study, application of TAT105 to various types of practical-scale composting treatments or composting of the wastes of other livestock species should be examined, and the effective use should be developed there.
Author contribution
K.K. conceived and designed the study. K.K., A.T., and K.F. performed the experiments. K.K. drafted the manuscript. K.N. offered counsels on the study and reviewed the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors are thankful to Mr Tohru Hikosaka, Mr Masuo Inokuchi, Mr Hidenori Nagano, Mr Yukinari Kawahara, Ms Hitomi Hisamatsu, and the late Mr Toshiharu Fujii for their assistance with the composting tests. We are also grateful to Dr. Yuji Kaji for his precise advice on contents and descriptions in this article.
References
- Finstein MS, Morris ML. Microbiology of municipal solid waste composting. Adv Appl Microbiol. 1975;19:113–151.10.1016/S0065-2164(08)70427-1
- F Zucconi, M. de Bertoldi. Compost specifications for the production and characterization of compost from municipal solid waste. In: M de Bertoldi, MP Ferranti, P L’Hermite, et al., editors. Compost: Production, Quality and Use. Barking: Elsevier Applied Science Publishers Ltd.; 1987. p. 30–50.
- K Haga. Production of compost from organic wastes. Extension Bull (ASPAC/FFTC) 1990;311:1–18.
- Y Harada. Composting and application of animal wastes.Extension Bull (ASPAC/FFTC) 1990;311:19–31.
- Haga K. Development of composting technology in animal waste treatment. Asian-Aust J Anim Sci. 1999;12:604–606.10.5713/ajas.1999.604
- Bernal MP, Alburquerque JA, Moral R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour Technol. 2009;100:5444–5453.10.1016/j.biortech.2008.11.027
- Kuroda K, Osada T, Yonaga M, et al. Emissions of malodorous compounds and greenhouse gases from composting swine feces. Bioresour Technol. 1996;56:265–271.10.1016/0960-8524(96)00047-8
- Osada T, Fukumoto Y. Development of a new dynamic chamber system for measuring harmful gas emissions from composting livestock waste. Wat Sci Technol. 2001;44:79–86.
- Fukumoto Y, Osada T, Hanajima D, et al. Patterns and quantities of NH3, N2O and CH4 emissions during swine manure composting without forced aeration – effect of compost pile scale. Bioresour Technol. 2003;89:109–114.10.1016/S0960-8524(03)00060-9
- Monteny GJ, Hartung E, editors. Ammonia emissions in agriculture. Wageningen: Wageningen Academic Publishers; 2007.
- Kuroda K, Hanajima D, Fukumoto Y, et al. Isolation of thermophilic ammonium-tolerant bacterium and its application to reduce ammonia emission during composting of animal wastes. Biosci Biotechnol Biochem. 2004;68:286–292.10.1271/bbb.68.286
- Kuroda K, Waki M, Yasuda T, et al. Utilization of Bacillus sp. strain TAT105 as a biological additive to reduce ammonia emissions during composting of swine feces. Biosci Biotechnol Biochem. 2015;79:1702–1711.10.1080/09168451.2015.1042831
- JM Bremner.Nitrogen-total. In: DL Sparks, PA Helmke, RH Loeppert, et al., editors. Methods of soil analysis. Part 3 chemical methods. Madison (WI): American Society of Agronomy; 1996. p.1085–1121.
- Bremner JM, Keeney DR. Steam distillation methods for determination of ammonium, nitrate, and nitrite. Anal Chim Acta. 1965;32:485–495.10.1016/S0003-2670(00)88973-4
- Zhu J. A review of microbiology in swine manure odor control. Agric Ecosys Environ. 2000;78:93–106.10.1016/S0167-8809(99)00116-4
- McCrory DF, Hobbs PJ. Additives to reduce ammonia and odor emissions from livestock wastes. J Environ Qual. 2001;30:345–355.10.2134/jeq2001.302345x
- Haga K. Managing manure on Japanese livestock and poultry farms. Biocycle. 2001;42:66.