Abstract
In this study, we examined the inhibitory effects of 14 food additives derived from polyphenol samples on staphylococcal enterotoxin A (SEA) production and biofilm formation by Staphylococcus aureus. Tannic acid AL (TA), Purephenon 50 W (PP) and Polyphenon 70A (POP) at 0.25 mg/mL and Gravinol®-N (GN), Blackcurrant polyphenol AC10 (BP), and Resveratrol-P5 (RT) at 1.0 mg/mL significantly decreased SEA production by S. aureus C-29 (p < 0.05). TA, GN, BP, and RT significantly inhibited the expression of the sea gene in S. aureus C-29 (p < 0.05), while suppression attempts by PP and POP proved unsuccessful. After result analysis, it can be derived that TA, GN, BP, and RT inhibit the production of SEA. Of the six samples, each one significantly inhibited biofilm formation (p < 0.05). Food additives derived from polyphenols have viability to be used as a means to inhibit the enterotoxin production and control the biofilm formation of foodborne pathogens.
Food additives derived from polyphenols inhibit staphylococcal enterotoxin A production and biofilm formation by Staphylococcus aureus.

Despite improvement in sanitary conditions in Japan, bacteria such as Staphylococcus aureus and Escherichia coli remain one of the leading causes of food poisoning. In general, antimicrobial ingredients are used to prevent foodborne illness. Although some antimicrobial ingredients are useful in reducing or eliminating S. aureus, antimicrobial ingredients may kill bacteria, such as Staphylococcus epidermidis, which are beneficial to human life. In addition, antimicrobial ingredients being used in abundance may result in the development of resistant strains. Therefore, the development of a control method to suppress the expression of pathogenic factors for preventing food poisoning is vital.
Staphylococcal food poisoning is caused by staphylococcal enterotoxins (SEs) produced by the growth of S. aureus. There are 23 types of SEs reported from SEA to SElX.Citation1,2) Among the SEs, more than 80% of staphylococcal food poisoning is caused by staphylococcal enterotoxin A (SEA).Citation3) Notably, SEA is active in low microgram quantities,Citation4) and is resistant to various conditions (heat treatment, low pH) that easily destroy the bacteria producing them, as well as proteolytic enzymes, hence retaining their toxic activity in the digestive tract after ingestion.Citation5–7) From the viewpoint of the prevention of staphylococcal food poisoning, methods which inhibit the production of virulence factors by S. aureus are necessary.
In recent years, the biofilm formation of food poisoning bacteria has acquired attention to due to the potential risks, including an enhanced level of antimicrobial resistance and the production of virulence factors leading to foodborne diseases. Some strategies have been used for controlling biofilm establishment in foods.Citation8) One approach is to use plant-derived natural products as a possible alternative to chemical methods of food preservation and biofilm formation on food surfaces.Citation9) Essential oils were reported to have components which showed inhibitory effects on biofilm formation of S. aureus.Citation10) Polyphenols are natural antioxidants in plant-derived natural products and show antimicrobial activities in food products.Citation11) Moreover, food additives derived from polyphenols are considered safe alternatives to synthetic compounds.
In the present study, we examined the inhibitory effect of food additives derived from polyphenols on SEA production and expression. In addition, further investigation into whether food additives derived from polyphenols inhibited the formation of biofilm was conducted.
Materials and methods
Bacterial strains
Each study utilized the SEA-producing strain S. aureus C-29.Citation12) The high-biofilm-forming strain S. aureus C-77 and S. aureus C-29 were used to examine the biofilm-forming ability. S. aureus C-29 and C-77 were isolated from human hands.Citation13) These bacteria were grown in a brain heart infusion (BHI; Difco Laboratories, Detroit, MI, USA) medium at 37 °C for 18 h while undergoing constant shaking.
Food additives derived from polyphenols
Fourteen different food additives derived from polyphenols were used (Table ). All food additives derived from polyphenols were provided according to San-Ei Gen F.F.I., Inc. (Osaka, Japan). The main component of tannic acid AL (TA; Fuji Chemical Industry, Co., Ltd., Wakayama, Japan) and purification kaki-tannin (PKT; Marine Science Co., Ltd., Tokyo, Japan) is tannic acid; purephenon 50 W (PP; Ogawa & Co., Ltd., Tokyo, Japan), sunfood 100 (SF; Mitsubishi-Chemical Foods Co., Ltd., Tokyo, Japan), and polyphenon 70A (PP; Mitsui Norin Co., Ltd., Tokyo, Japan) are tea catechins; grape extract HA (GE; San-Ei Gen F.F.I., Inc.) and gravinol®-N (GN; Kikkoman Biochemifa Co., Tokyo, Japan) are proanthocyanidins; blackcurrant polyphenol AC10 (BP; Meiji Food Materia Co., Ltd., Tokyo, Japan) are anthocyanins; applin AFPOMM9051 (APL; Unitec Foods Co., Ltd., Tokyo, Japan) is apple polyphenol; resveratrol-P5 (RT; Oriza Oil & Fat Chemical Co., Ltd., Aichi, Japan) and resveratrol-WSP 0.5 (RT-0.5; Oriza Oil & Fat Chemical Co., Ltd., Aichi, Japan) are resveratrols; green coffee bean extract-P (GCBE; Oriza Oil & Fat Chemical Co., Ltd., Aichi, Japan) is chlorogenic acid; walnut polyphenol-P30 (WP; Oriza Oil & Fat Chemical Co., Ltd., Aichi, Japan) is walnut polyphenol; evening primrose extract-P (EPE; Oriza Oil & Fat Chemical Co., Ltd., Aichi, Japan) is gallic acid.
Table 1. Food additives derived from polyphenols used in this study.
Influence of food additives derived from polyphenols on the growth of S. aureus
Each food additive derived from polyphenols was diluted in dimethyl sulfoxide. A stock culture of the S. aureus C-29 was inoculated into a BHI medium at 37 °C for 19 h while undergoing constant shaking. This culture fluid (about 108–109 CFU mL) was diluted with sterilized PBS to a final concentration of 103–104 CFU/mL and further diluted with 2 × BHI medium to a final concentration of 102–103 CFU/mL. Each food additive derived from polyphenols (final concentration of 1.0 and 2.5 mg/mL) was added to this diluted solution and incubated at 37 °C for 1 h. Each bacterial suspension was diluted 10 times and then 100 μL of each dose/bacterial suspension was plated onto a mannitol salt plate (Eiken Chemical, Tokyo, Japan). Inoculates agar plates were incubated at 37 °C for 48 h, and then colony-forming units were determined.
SDS-PAGE and Western blot analysis
The interaction between each food additive derived from polyphenols and SEA-producing strain S. aureus C-29 was estimated as previously described.Citation12,14) A stock culture of the S. aureus C-29 was inoculated into a BHI medium at 37 °C for 19 h while undergoing constant shaking. The cultured fluid (about 108–109 CFU mL) was diluted with sterilized PBS to a final concentration of 103–104 CFU/mL and further diluted with 2 × BHI medium to a final concentration of 102–103 CFU/mL. Each food additive derived from polyphenols (final concentration of 0.1, 0.25, 0.5, 1.0, 2.5 mg/mL) was added to this diluted solution. The solution was then incubated at 37 °C for 24 h. The Western blot analysis was previously described in detail.Citation12) The separation of the resultant supernatants was performed on 15% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membranes were blocked in phosphate-buffered saline containing 0.05% Tween 20 and blocked with 0.6% skim milk for 2 h and incubated at 4 °C overnight with rabbit anti-SEA IgG (Sigma, St. Louis, Mo., USA), followed by incubation for 1 h with goat anti-rabbit peroxidase (KPL). The immunoreactive bands were detected using a BCIP/NBT substrate (KPL) and quantified using Image J software (Natl. Inst. of Health, Bethesda, Md., USA).
RNA isolation and real-time RT-PCR
A stock culture of S. aureus C-29 was inoculated into a BHI medium at 37 °C for 19 h while undergoing constant shaking. This culture fluid (about 108–109 CFU mL) was diluted with sterilized PBS to a final concentration of 103–104 CFU/mL and further diluted with 2 × BHI medium to a final concentration of 102–103 CFU/mL. Each food additive derived from polyphenols (final concentration of TA, PP, and POP; 0.3, 0.4, 0.5 mg/mL, GN, BP, and RT; 1.0, 2.0, 2.5 mg/mL) was added to this diluted solution and incubated at 37 °C for 6 h. RNA extraction was performed using a RiboPure-Bacteria kit (Ambion, Austin, TX, USA), in accordance with the manufacturer’s instructions. The real-time PCR was performed using a SYBR Premix Ex Taq (Takara, Japan) and a real-time PCR system (Thermal Cycler Dice® Real Time System Single; Takara). Each sample was normalized to 16S rRNA, triplicates were averaged, and relative mRNA levels were determined. Each sample was normalized to 16S rRNA, and relative mRNA levels were determined. The following primers were used for the RT-PCR: sea F, 5′-AAAATACAGTACCTTTGGAAACGGTT-3′ and sea R, 5′-TTTCCTGTAAATAACGTCTTGCTTGA-3′; 16S rRNA F, 5′-GCGAAGAACCTTACCAAATC-3′ and 16S rRNA R, 5′-CCAACATCTCACGACACG-3′.
Crystal violet assay
Each stock culture of S. aureus C-29 and high-biofilm-forming strain S. aureus C-77 was inoculated into a BHI medium at 37 °C for 6 h while undergoing constant shaking. Each 10 μL of these two cultured fluids (approximately 108–109 CFU mL) and each food additive derived from polyphenols (final concentration of TA, PP, and POP; 0.3, 0.4, 0.5 mg/mL, GN, BP, and RT; 1.0, 2.0, 2.5 mg/mL) was added to a 66% tryptic soy broth (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) containing 0.2% glucose in 96-well tissue culture plates. After completion of an incubation conducted for 48 h at 37 °C, Planktonic bacteria were cleansed from the wells via sterile distilled water and then 125 μL 0.1% crystal violet solution was added and incubated for 10 min at room temperature. The wells were eliminated of excessive crystal violet stains via three rinsing with sterile distilled water and then air-dried for 10 min at room temperature. Following this procedure, 100 μL of 99.5% ethanol was added to test wells for 10 min for the purpose of destaining the wells. Biofilm formation was quantified by measuring the absorbance values using a microplate reader at 595 nm. Relative biofilm formation was defined as follows:
Statistical analysis
The results were analyzed using a Student t-test or one-way ANOVA, followed by the Dunnett’s test using Microsoft Excel 2013 (Microsoft, Redmond, WA, USA). The significance level was set at p < 0.05 or < 0.01, and each experiment underwent a minimum of three replications.
Results
Influence of food additives derived from polyphenols on the growth of S. aureus
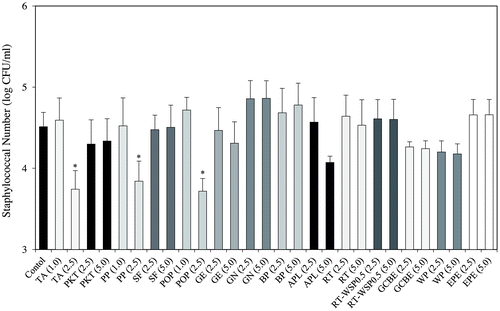
Utilizing the dilution plate method, food additives derived from polyphenols on S. aureus growth were investigated in order to determination the effects displayed on bacterial CFU. The test was conducted with a maximum sample concentration of 5.0 mg/mL. When sample concentrations were less than 1.0 mg/mL (TA, PP, and POP) and 5.0 mg/mL (PKT, SF, GE, GN, BP, APL, RT, RT-WSP0.5, GCBE, WP, and EPE), no effect on S. aureus growth (Fig. ) was observed. All following assays were performed at non-growth inhibitory concentrations.
Fig. 1. Antimicrobial effects of food additives derived from polyphenols on SEA-producing strain Staphylococcus aureus C-29.
Inhibitory effect of food additives derived from polyphenols on SEA production
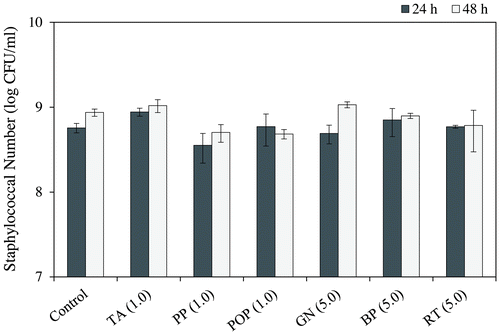
Food additives inhibitory effects derived from polyphenols on SEA production were examined using the Western blot analysis. As a result, TA, PP, and POP at 0.25 mg/mL and GN, BP, and RT at 1.0 mg/mL significantly decreased the band intensity of SEA protein (p < 0.05) (Fig. ). Other samples (PKT, SF, GE, APL, RT-WSP0.5, GCBE, WP, and EPE) exhibited no change in the protein band of SEA at the concentration of 5.0 mg/mL. In the six samples (TA, PP, POP, GN, BP, and RT) which decreased the protein band of SEA, bacteria which displayed a significantly different number from the control group after incubation for 24 h were nonexistent (MilliQ water) (Fig. ). Given the possibility for these six samples to directly react with SEA, we examined whether they could suppress the expression level of the SEA toxin-encoding gene (sea gene).
Fig. 2. Interaction between food additives derived from polyphenols and cultured staphylococcal enterotoxin A (SEA)-producing strain.
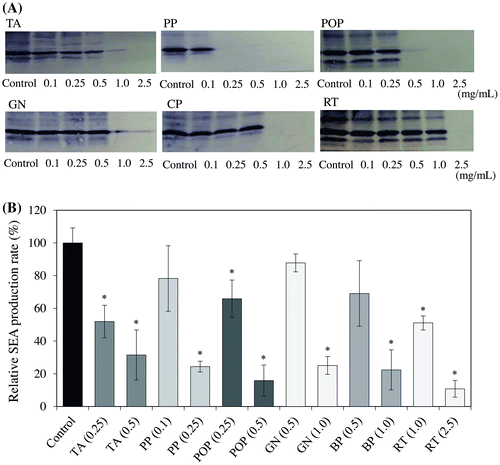
Fig. 3. Growth effect of food additives derived from polyphenols on Staphylococcus aureus.
Inhibitory effect of food additives derived from polyphenols on SEA gene expression
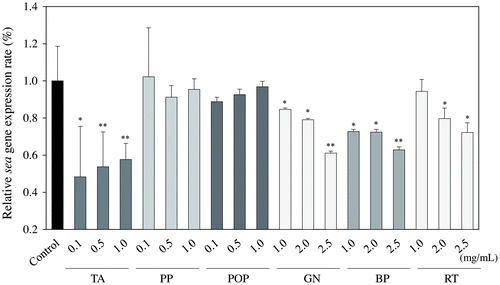
Based on the findings that TA, PP, POP, GN, BP, and RT inhibited the production of SEA, we used a real-time RT-PCR assay to further investigate the relative expression level of the sea gene after treatment with these samples. As shown in Fig. , TA (0.3 mg/mL), GN (1.0 mg/mL), BP (1.0 mg/mL), and RT (2.0 mg/mL) significantly inhibited the expression of SEA in S. aureus C-29 at the logarithmic growth phase (6 h-old culture) (p < 0.05). These results approximate that due to the complexity of SEA and PP or POP being precipitated, SEA was not detected from the culture supernatant.
Fig. 4. Relative gene expression of sea in Staphylococcus aureus C-29 after incubation with food additives derived from polyphenols.
Inhibitory effect of food additives derived from polyphenols on biofilm formation
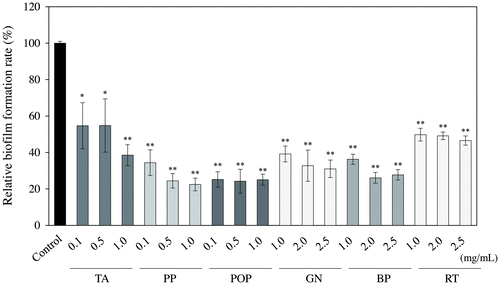
The inhibitory effects of the six samples (TA, PP, POP, GN, BP, and RT), that decreased the protein band of SEA, on staphylococcal biofilm formation were evaluated. In these six samples, the number of bacteria after incubation for 48 h exhibited no significant difference from the control (MilliQ water) (Fig. ). As shown in Fig. , each sample significantly inhibited the biofilm formation at a concentration which did not affect the growth of S. aureus (p < 0.05).
Fig. 5. Inhibitory effect of food additives derived from polyphenols on biofilm formation.
Discussion
The applications of plant extracts or derivatives in various foods and beverages are increasing effervescently in the food industry. In this study, the inhibitory effects of 14 food additives derived from polyphenol samples on SEA production and biofilm formation were examined. Initially, the effect of food additives derived from polyphenols on S. aureus growth was hypothesized. As a result of testing with the maximum concentration of 5.0 mg/mL, the growth of S. aureus was suppressed at 2.5 mg/mL in three samples (TA, PP, and POP) (Fig. ). Other samples showed no inhibition of the growth of S. aureus at 5.0 mg/mL, suggesting that the antibacterial effect of these samples is weak. Tannic acid, the main component of TA, is present in many foods, e.g., tea, cocoa beans, coffee beans, and persimmon. Tannic acid is also categorized as a “generally recognized as safe” (GRAS) food additive.Citation15) Tannic acid has long been known to have antibacterial properties.Citation16) Nonetheless, it was reported that tannic acid at 20 μM does not inhibit the growth of S. aureus.Citation17) As tannic acid does not have a strong antibacterial action, it is not used as a food additive for the purpose of controlling bacteria.
On the other hand, phenolic extracts prepared from green tea are known to have antimicrobial effects against foodborne pathogens.Citation18) Green tea extract, which is the main component of PP and POP, is the plant extract popularized for its varied use in food and beverage applications. The green tea extract has a peculiar flavor and reacts with iron ions to provide color easily, and thus the concentration that can be added without affecting the flavor and appearance of the food is about 100 ppm. From such characteristics, green tea extract is not used as an antimicrobial agent but as an antioxidant. Due to this, all assays were performed at non-growth inhibitory concentrations.
The effect of each food additive derived from a polyphenol sample on the SEA-producing strain of S. aureus C-29 was examined using the Western blot analysis. As a result, TA, PP, and POP at 0.25 mg/mL and GN, BP, and RT at 1.0 mg/mL significantly decreased the band of SEA protein produced by S. aureus C-29 (p < 0.05) (Fig. ). These results suggest that TA, PP, POP, GN, BP, and RT inhibit the production of SEA from S. aureus C-29. Further investigation was conducted into the relative expression level of the sea gene after treatment with these six samples using a real-time RT-PCR assay. In turn, TA, GN, BP, and RT were derived to significantly inhibit the expression of the sea gene in S. aureus C-29 (p < 0.05), while PP and POP did not suppress the expression of the sea gene (Fig. ). These results suggest that TA, GN, BP, and RT inhibit the production of SEA, and PP and POP may react directly with SEA. It is thought that SEA was not detected in the culture supernatant due to precipitation of PP and POP by direct reaction with SEA. The phenolic compounds including eugenol, and the terpenoids including citronellol and geraniol, had a significant influence on SEA production.Citation19) Therefore, we suggested that polyphenols contained in food additives had an inhibitory effect on SEA production. Further studies are needed as to whether polyphenols in PP and POP react directly to SEA.
Phenolic compounds have an inhibitory effect on biofilm formation by affecting bacterial regulatory mechanisms, such as quorum sensing or other global regulator systems, without any effect on bacterial growth.Citation20) The S. aureus anti-biofilm effect was present in several phenolic acids, including gallic,Citation21) ellagic,Citation22) ginkgolicCitation23), and rosmarinic acidsCitation24) at sub-inhibitory concentrations. Therefore, the inhibitory effects of TA, PP, POP, GN, BP, and RT, which decreased the protein band of SEA, on staphylococcal biofilm formation, were evaluated. As a result, all samples significantly inhibited the biofilm formation at a concentration which did not affect the growth of S. aureus (p < 0.05) (Fig. ). Comparisons of each sample at 1.0 mg/mL led to the discovery of the biofilm formation inhibition rate being 50, 61, 62, 64, 75, and 76% at RT, GN, TA, BP, POP, and PP, respectively. PP and POP, which have tea catechins as their main component, showed a strong ability to inhibit biofilm formation. It was reported that sub-MICs of epigallocatechin gallate (EGCg), the main polyphenol component of green tea inhibits biofilm formation in S. aureus. EGCG is known to bind to peptidoglycan, leading to disruption of the integrity of the bacterial cell wall.Citation25,26) Taking this into consideration, EGCG was able to interfere with the earlier stages of biofilm formation, which requires hydrophobic interactions between the bacterial cell wall and the surface to be colonized.Citation27) Pentagalloyl glucose (one of the major components of commercial tannic acid) and ellagic acid (the polyphenolic compound derived from a species of wild blackberry) have been shown to inhibit biofilm formation in S. aureus.Citation28,29) Moreover, cranberries contain proanthocyanidin which exhibit weak biofilm eradication of the Staphylococcus species with minimum biofilm eradication concentration values of 5–10 mg/mL.Citation30) Thus, it is known that select polyphenols have an inhibitory effect on S. aureus biofilm formation.Citation27–30) These reports and our results suggested that the action of polyphenols contained in food additives may inhibit biofilm formation at concentrations not inhibiting the growth of S. aureus. However, future examinations of various S. aureus strains are necessary.
Polyphenols are not only used as food additives around the world, but also improve the sensory properties of foods and extend the shelf life via their antioxidant action.Citation31) Furthermore, polyphenols have been not used as antimicrobial ingredients as they affect the color and taste of foods when used in high concentrations. In particular, one of the prominent sensory characteristics of polyphenols is bitterness.Citation32) The results of this study revealed that low concentrations of food additives derived from polyphenols which show no antimicrobial activity and do not affect food taste have inhibitory effects on toxin production and biofilm formation of S. aureus. Therefore, they may show the inhibitory effects at a lower concentration than that used in food. We could propose the future use of food additives derived from polyphenols to inhibit enterotoxin production and control the biofilm of the foodborne pathogens in food. Further research is needed to use the food additives derived from polyphenols for inhibition of bacterial pathogenic factors as well as to examine the combination effects with other agents.
Author contribution
Yuko Shimamura, Masatsune Murata, and Shuichi Masuda conceived and designed the experiments; Yuko Shimamura, Chikako Hirai, Yuka Sugiyama, Masaharu Shibata, and Junya Ozaki performed the experiments; Yuko Shimamura, Norio Ohashi, and Shuichi Masuda analyzed the data; Yuko Shimamura wrote the paper.
Funding
This study was supported in part by JSPS KAKENHI [grant number 26560061]; grant from the Japan Food Chemical Research Foundation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wilson GJ, Seo KS, Cartwright RA, et al. A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLoS Pathog. 2011;7:e1002271.10.1371/journal.ppat.1002271
- Hu DL, Nakane A. Mechanisms of staphylococcal enterotoxin-induced emesis. Eur J Pharmacol. 2014;722:95–107.10.1016/j.ejphar.2013.08.050
- Balaban N, Rasooly A. Staphylococcal enterotoxin. Int J Food Microbiol. 2000;61:1–10.10.1016/S0168-1605(00)00377-9
- Larkin EA, Carman RJ, Krakauer T, et al. Staphylococcus aureus: the toxic presence of a pathogen extraordinaire. Curr Med Chem. 2009;16:4003–4019.10.2174/092986709789352321
- Asao T, Kumeda Y, Kawai T, et al. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol Infec. 2003;130(1):33–40.10.1017/S0950268802007951
- Evenson ML, Hinds MW, Bernstein RS, et al. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Int J Food Microbiology. 1988;7:311–316.10.1016/0168-1605(88)90057-8
- Schantz EJ, Roessler WG, Wagman J, et al. Purification of staphylococcal enterotoxin B. BiochemistryBiochem. 1965;4:1011–1016.10.1021/bi00882a005
- Alves D, Olívia Pereira M. Mini-review: antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling. 2014;30(4):483–499.10.1080/08927014.2014.889120
- Leonard CM, Virijevic S, Regnier T, et al. Bioactivity of selected essential oils and some components on Listeria monocytogenes biofilms. S Afr J Bot. 2010;76(4):676–680.10.1016/j.sajb.2010.07.002
- Jafri H, Husain FM, Ahmad I. Antibacterial and antibiofilm activity of some essential oils and compounds against clinical strains of Staphylococcus aureus. J Biomed Ther Sci. 2014;1(1):65–71.
- Perumalla AVS, Hettiarachchy NS. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res Int. 2011;44(4):827–839.10.1016/j.foodres.2011.01.022
- Shimamura Y, Aoki N, Sugiyama Y, et al. Screening of tea extract and theaflavins for inhibitory effects on the biological activity and production of staphylococcal enterotoxin A. J Food Sci. 2014;79:M2294–M2300.10.1111/1750-3841.12566
- Shimamura Y, Kidokoro S, Murata M. Survey and properties of Staphylococcus aureus isolated from Japanese-style desserts. Biosci Biotechnol Biochem. 2006;70(7):1571–1577.10.1271/bbb.50617
- Shimamura Y, Aoki N, Sugiyama Y, et al. Plant-derived polyphenols interact with staphylococcal wnterotoxin A and inhibit toxin activity. PLoS ONE. 2016;11:e0157082.10.1371/journal.pone.0157082
- Chung KT, Stevens SE Jr, Lin WF, et al. Growth inhibition of selected food-borne bacteria by tannic acid, propyl gallate and related compounds. Lett Appl Microbiol. 1993;17:29–32.10.1111/j.1472-765X.1993.tb01428.x
- Henis Y, Tagari H, Volcani R. Effect of water extracts of carob pods, tannic acid, and their derivatives on the morphology and growth of microorganisms. Appl Microbiol. 1964;12(3):204–209.
- Payne DE, Martin NR, Parzych KR, et al. Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner. Infect Immun. 2013;81(2):496–504.10.1128/IAI.00877-12
- An BJ, Kwak JH, Son JH, et al. Biological and anti-microbial activity of irradiated green tea polyphenols. Food Chem. 2004;88(4):549–555.10.1016/j.foodchem.2004.01.070
- Albano M, Alves FCB, Andrade BFMT, et al. Antibacterial and anti-staphylococcal enterotoxin activities of phenolic compounds. Innov Food Sci Emerg Technol. 2014;38:83–90.
- Silva LN, Zimmer KR, Macedo AJ, et al. Plant natural products targeting bacterial virulence factors. Chem Rev. 2016;116:9162–9236.10.1021/acs.chemrev.6b00184
- Luis A, Silva F, Sousa S, et al. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling. 2014;30:69–79.10.1080/08927014.2013.845878
- Bakkiyaraj D, Nandhini JR, Malathy B, et al. The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling. 2013;29:929–937.10.1080/08927014.2013.820825
- Lee JH, Kim YG, Ryu SY, et al. Ginkgolic acids and Ginkgo biloba extract inhibit Escherichia coli O157:H7 and Staphylococcus aureus biofilm formation. Int J Food Microbiol. 2014;174:47–55.10.1016/j.ijfoodmicro.2013.12.030
- Slobodníková L, Fialová S, Hupková H, et al. Rosmarinic acid interaction with planktonic and biofilm Staphylococcus aureus. Nat Prod Commun. 2013;8:1747–1750.
- Yoda Y, Hu ZQ, Shimamura T, et al. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J Infect Chemother. 2004;10:55–58.10.1007/s10156-003-0284-0
- Zhao WH, Hu ZQ, Okubo S, et al. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1737–1742.10.1128/AAC.45.6.1737-1742.2001
- Carpentier B, Cerf O. Biofilms and their consequences, with particular reference to hygiene in the food industry. J Appl Bacteriol. 1993;75:499–511.10.1111/j.1365-2672.1993.tb01587.x
- Lin MH, Chang FR, Hua MY, et al. Inhibitory effects of 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucopyranose on biofilm formation by Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55(3):1021–1027.10.1128/AAC.00843-10
- Quave CL, Estévez-Carmona M, Compadre CM, et al. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE. 2011;7(1):e28737.
- LaPlante KL, Sarkisian SA, Woodmansee S, et al. Effects of cranberry extracts on growth and biofilm production of Escherichia coli and Staphylococcus species. Phytother Res. 2012;26(9):1371–1374.10.1002/ptr.v26.9
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects – a review. J Funct foods. 2015;18:820–897.10.1016/j.jff.2015.06.018
- Stein LJ, Nagai H, Nakagawa M, et al. Effects of repeated exposure and health-related information on hedonic evaluation and acceptance of a bitter beverage. Appetite. 2003;40:119–129.10.1016/S0195-6663(02)00173-3
