Abstract
In the present study, formaldehyde dismutase from Methylobacterium sp. FD1 was partially purified and analyzed by nanoLC–MS/MS; it was then cloned from the genomic DNA of FD1 by PCR. The open reading frame of the formaldehyde dismutase gene of FD1 was estimated to be 1203 bp in length. The molecular weight and pI of formaldehyde dismutase (401 aa), as deduced from the FD1 gene, were calculated at 42,877.32 and 6.56, respectively. NAD(H)-binding residues and zinc-binding residues were found in the amino acid sequence of the deduced formaldehyde dismutase of FD1 by BLAST search. The resting Escherichia coli cells that were transformed with the FD1 formaldehyde dismutase gene degraded high concentrations of formaldehyde and produced formic acid and methanol that were molar equivalents of one-half of the degraded formaldehyde. The lyophilized cells of the recombinant E. coli also degraded high concentrations of formaldehyde.
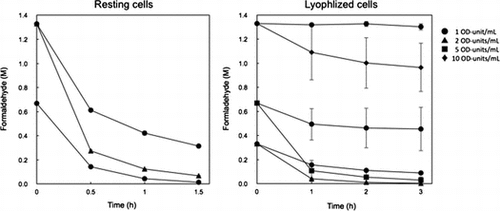
Degradation of formaldehyde by resting and lyophilized cells of E. coli transformed with the formaldehyde dismutase gene of Methylobacterium sp. FD1.
Formaldehyde (HCHO) is widely used as a raw material in a variety of chemical products, such as resins, as well as a preservative for cosmetics, detergents, and human cadavers and animal organs for research. Degradation of formaldehyde is necessary to prevent environmental pollution. Although a number of technologies are available to remove or degrade formaldehyde in its liquid pollution, biological methods are desirable because they are safe and relatively inexpensive. In our preceding paper [Citation1], we described the isolation of a formaldehyde-degrading bacterium, Methylobacterium sp. FD1, from soil; the resting cells of FD1 degraded high concentrations of formaldehyde (~2.7 M) and produced to formic acid and methanol in molar equivalents of one-half of the degraded formaldehyde. These results suggested that the formaldehyde degradation enzyme of FD1 was formaldehyde dismutase (EC 1.2.99.4). Formaldehyde dismutase is known to catalyze the dismutation of formaldehyde in a ping-pong mechanism without the addition of coenzyme because the enzyme is a nicotinoprotein that contains nicotinamide adenine dinucleotide (NAD(H)) as a tightly bound, redox-active cofactor [Citation2,3]. For the industrial use of formaldehyde dismutase or of the bacterial cells producing the enzyme, it is advantageous to not require an expensive coenzyme for the degradation of high concentrations of formaldehyde. The lyophilized cells of FD1 that were already dead also degraded high concentrations of formaldehyde [Citation1]. We think that the lyophilized dead cells of FD1 are available for formaldehyde degradation in the laboratory or field without microbial contamination. The productivity of FD1 cells with formaldehyde dismutase activity was relatively low because of their low growth rate (doubling time: 8.4 h at 30 °C with shaking in LB medium). In general, Escherichia coli have a relatively high growth rate and are good hosts for the production of heterologous proteins. Therefore, we proposed to degrade formaldehyde using E. coli transformed with the formaldehyde dismutase gene of FD1. Formaldehyde dismutase has been purified from and characterized in only Pseudomonas putida F61 [Citation3,4], and formaldehyde dismutase genes have been cloned only from P. putida F61 and J3 [Citation5,6]. The glutathione-independent formaldehyde dehydrogenase of P. putida C83 is functionally identical to the formaldehyde dismutase of P. putida F61 [Citation2]. The glutathione-independent formaldehyde dehydrogenase of P. putida C83 has also been purified and characterized [Citation2,7] and its gene has been cloned [Citation8]. In the present study, we partially purified the formaldehyde dismutase of FD1, cloned the formaldehyde dismutase gene of FD1, and found that the resting and lyophilized cells of E. coli transformed with the FD1 formaldehyde dismutase gene degraded high concentrations of formaldehyde.
Materials and methods
Chemicals
Formaldehyde solution (37% [w/v], containing 5–10% [w/v] methanol) and kanamycin were purchased from Wako Pure Chemical Industries (Osaka, Japan). Formaldehyde solution (16% [w/v], methanol-free) was purchased from Thermo Fisher Scientific (Rockford, IL, USA). Bacto™ Tryptic Soy Broth (Soybean–Casein Digest Medium), yeast extract, and Bacto™ Tryptone (Enzymatic digest of casein) were purchased from Becton, Dickinson and Company (Sparks, MD, USA).
Bacterial strains and plasmid
Methylobacterium sp. FD1 [Citation1] cells were used for the extraction of the enzyme and genomic DNA. Expresso® Rhamnose Cloning and Expression System (Lucigen, Middleton, WI, USA) containing the host E. coli strain E. cloni® G10 and the expression plasmid pRham™ C-His Kan vector carrying L-rhamnose-inducible rhaPBAD promoter [Citation9] was used for the expression of the heterologous protein.
Partial purification of formaldehyde dismutase of Methylobacterium sp. FD1
Extraction of enzyme: FD1 cells were cultured at 30 °C for 3 days with shaking in tryptic soy broth. The cells in the culture (1 L) were harvested by centrifugation at 14,350 ×g at 4 °C for 3 min, rinsed with 0.1 M potassium phosphate buffer (pH 7), then suspended in the same buffer. The cells were disrupted with an ultrasonic disrupter (UD-211; TOMY SEIKO, Tokyo, Japan) at 20 kHz for 30 min (2 min × 15 times) on ice. The sonicated samples were centrifuged at 32,300 ×g at 4 °C for 15 min. The resulting supernatant (cell-free protein extract) was stored at −25 °C.
Ammonium sulfate fractionation: Solid ammonium sulfate was added to the cell-free protein extract from FD1 to yield a 30% saturated solution and the mixture was kept on ice for 30 min. After centrifugation at 32,300 ×g at 4 °C for 30 min, the precipitate was dissolved in 10 mM potassium phosphate buffer (pH 7) (30%-fraction). For the supernatant, additional ammonium sulfate was added, yielding a 40% saturated solution. The precipitation procedure was repeated to produce the 90% fraction.
Hydrophobic interaction chromatography: The fractions obtained by ammonium sulfate fractionation with formaldehyde dismutase activity were mixed and solid ammonium sulfate was added to the mixture to 1.5 M. An aliquot (2 mL) of this mixture containing 1.5 M ammonium sulfate was applied to a HiPrep™ Phenyl FF column (1.6 × 10 cm) (GE Healthcare Biosciences, Piscataway, NJ, USA) equilibrated with 50 mM potassium phosphate buffer (pH 7) containing 1.5 M ammonium sulfate. After the column was washed with 100 mL of the same buffer, the adsorbed enzymes were eluted with a linear gradient of decreasing ammonium sulfate concentration (final concentration: 0, total volume: 305 mL) at a flow rate of 2 mL/min and each 3-mL fraction was collected (total of 135 fractions).
Simple measurement of formaldehyde dismutase activity
The fractions from the ammonium sulfate fractionation were mixed with 0.2% formaldehyde solution and incubated at 30 °C for 3 h. The reaction was diluted with distilled water and 0.1% methyl orange was added to the dilution. For the fractions from the hydrophobic interaction chromatography, each fraction and 0.4% formaldehyde solution were mixed and incubated at 30 °C for 3 h. Then, 0.1% methyl orange was added to the reaction and its absorbance (λ = 507 nm, A507) was measured. Formaldehyde dismutase activities were expressed as relative values (%) of A507 calibrated using a blank, which changed depending on the quantity of formic acid produced from formaldehyde by formaldehyde dismutase.
Preparation of protein extracts and precipitate fractions of recombinant E. coli
E. coli cells were suspended in 0.1 M sodium phosphate buffer (pH 7.8) containing 0.3 M NaCl, and then disrupted with an ultrasonic disrupter at 20 kHz for 150 s (15 s × 10 times) on ice. The sonicated samples were centrifuged at 20,630 × g at 4 °C for 15 min. The supernatant (cell-free protein extract) was used in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The precipitate was resuspended in 6 M urea, incubated at room temperature for 1 h, and centrifuged. The supernatant containing re-dissolved precipitate was analyzed by SDS-PAGE.
SDS-PAGE and nanoscale liquid chromatography coupled to tandem mass spectrometry (nanoLC–MS/MS) analysis
Enzyme samples were analyzed by SDS-PAGE using 10–20% polyacrylamide gradient gels (e-PAGEL, ATTO, Tokyo, Japan) in Tris-glycine electrophoresis buffer. Gels were stained with PageBlue™ Protein Staining Solution (Thermo Scientific, Rockford, IL, USA) for protein visualization. Protein bands were excised from the gels, digested with trypsin, and subjected to nanoLC–MS/MS analysis using a standard protocol. The data generated by nanoLC–MS/MS were subjected to search with the Mascot algorithm (Matrix-Science, London, UK).The nanoLC–MS/MS analysis and Mascot search were performed by Japan Proteomics (Sendai, Japan).
Polymerase chain reaction (PCR) amplification of the formaldehyde dismutase gene of Methylobacterium sp. FD1
Genomic DNA was extracted from FD1 cells cultured in tryptic soy broth using NucleoSpin® Tissue (MACHEREY-NAGEL, Düren, Germany), according to the manufacturer’s instructions. The partial fragment (the middle region) of the formaldehyde dismutase gene of FD1 was amplified by PCR using the genomic DNA of FD1 as a template and the primer pair FDM-mid-F1 and FDM-mid-R1 (Table ), which are designed based on the amino acid sequences of the peptides obtained by nanoLC–MS/MS analysis, with 30 cycles each of 98 °C for 10 s, 60 °C for 30 s, and 72 °C for 60 s. The flanking regions (the upstream and downstream regions) of the middle region of the formaldehyde dismutase gene of FD1 were amplified by thermal asymmetric interlaced PCR (TAIL-PCR) reported by Liu et al. [Citation10], using the genomic DNA of FD1 as a template. The full-length formaldehyde dismutase gene of FD1 was amplified by PCR using the genomic DNA of FD1 as a template and the primer pair FD1-FDM-F and FD1-FDM-R (Table ). The amplified DNA fragments were separated by agarose gel electrophoresis and purified using a QIAquick® Gel Extraction Kit (QIAGEN, Hilden, Germany).
Table 1. Primers for PCR amplification of the formaldehyde dismutase gene of FD1.
Construction of the plasmid
The recombinant plasmid (named pRham-FDM) ligated the full-length formaldehyde dismutase gene of FD1 under rhamnose promoter was constructed in E. coli strain E. cloni® G10 according to the manual of Expresso® Rhamnose Cloning and Expression System. Plasmids were purified using a Plasmid Mini Kit (QIAGEN, Hilden, Germany).
DNA sequencing and homology search
The purified DNA fragment and plasmid were sequenced using the dideoxynucleotide chain-termination method by an automated sequencer (ABI PRISM 310 Genetic Analyzer, Applied Biosystems, Foster City, CA, USA). Part of the DNA-sequencing was performed by RIKEN GENESIS (Chiba, Japan). DNA sequences and amino acid sequences were searched via the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov).
Formaldehyde degradation by resting and lyophilized recombinant E. coli cells
The recombinant E. coli cells were cultured in LB medium containing 30 μg/mL kanamycin at 37 °C for 13–15 h with shaking. The cultures were inoculated into LB medium containing 30 μg/mL kanamycin, 0.2% rhamunose and 0.05% glucose to an optical density at 660 nm (OD660) of 0.3–0.4 on a TAITEC spectrophotometer miniphoto 518R (TAITEC, Saitama, Japan) and cultured at 37 °C for 8 h with shaking. The cultures were diluted with saline to an optical density at OD660 of 1.0 and centrifuged at 14,400 ×g at 4 °C for 3 min. The cell pellet was rinsed with distilled water and centrifuged, then discard the supernatant (resting cells). The resting cells were frozen at −80 °C and lyophilized using the Shimadzu freeze dryer FDS-50 (Shimadzu, Kyoto, Japan) (lyophilized cells).
Resting and lyophilized cells of recombinant E. coli were suspended in 1 M potassium phosphate buffer (pH 7) containing 0.33 or 0.67 M formaldehyde, or in 1.4 M potassium phosphate buffer (pH 7) containing 1.33 M formaldehyde and incubated at 30 °C for 0.5–3 h. After incubation, formaldehyde concentration and colony forming units (CFU) were measured. Formaldehyde degradation activity was expressed as units/OD-unit. One unit corresponds to the amount of enzyme which degrades 1 μmol of formaldehyde in one minute in 1 M potassium phosphate buffer (pH 7) at 30 °C. OD unit was defined as the amount of bacteria in 1 mL of culture at OD660 of 1.0.
Analytical methods
Formaldehyde, formic acid, and methanol were determined as described previously [Citation1]. Protein was measured using a Protein Assay BCA kit (Wako Pure Chemical Industries, Osaka, Japan).
Nucleotide sequence accession number
The nucleotide sequence of the formaldehyde dismutase gene of FD1 has been submitted to the DNA Data Bank of Japan under the accession number LC269930.
Results and discussion
Partial purification and nanoLC–MS/MS analysis of formaldehyde dismutase from Methylobacterium sp. FD1
To purify FD1 formaldehyde dismutase, we subjected cell-free protein extracts of the FD1 cells to ammonium sulfate fractionation and measured the formaldehyde dismutase activities of the fractions with the simple assay described in Materials and methods. We found that the 50-, 60-, and 70%-fractions had relatively high formaldehyde dismutase activity (data not shown). These fractions were mixed and the mixture was subjected to hydrophobic interaction chromatography. As shown in Figure , fraction numbers 71–79 (A-fraction) and fraction numbers 89–93 (B-fraction) had relatively high formaldehyde dismutase activity.
Figure 1. Hydrophobic interaction chromatography of FD1 formaldehyde dismutase. The solid line, relative formaldehyde dismutase activity; the dashed line, the concentration of ammonium sulfate. (A) and (B) were the fractions with relatively high formaldehyde dismutase activity. Experimental conditions are specified in Materials and methods.
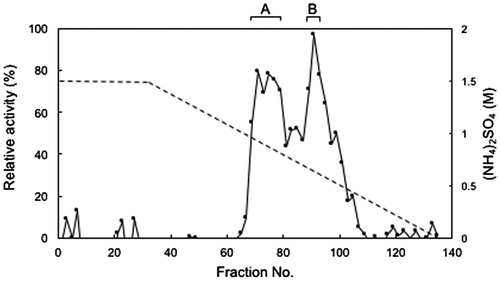
SDS-PAGE of A-fraction and B-fraction, obtained by hydrophobic interaction chromatography, showed two main bands at the positions corresponding to approximately 43 kDa (A43) and 41 kDa (A41) in A-fraction and three main bands corresponding to approximately 60 kDa (B60), 43 kDa (B43), and 36 kDa (B36) in B-fraction (Figure ). To assess if formaldehyde dismutase resided in these bands, we subjected them to nanoLS–MS/MS analysis and Mascot search. We found that 22 peptides (of which 7 were unique) from A43 and 16 peptides (of which 7 were unique) from A41 matched the aldehyde dismutase (glutathione-independent formaldehyde dehydrogenase) of Cupriavidus sp. SK-3 (protein code gi 917041957, accession No. WP_051648669), which is a putative enzyme identified using complete genome sequencing (Table ). The seven unique peptides from A43 were the same as those from A41 (data not shown). These results suggested that A43 and A41 contained formaldehyde dismutases, that the possible formaldehyde dismutases of A43 and A41 were the same, and that the enzyme was spread in the vicinity of 43–41 kDa on SDS-PAGE. Four peptides (of which two were unique) from B43 matched the glutathione-independent formaldehyde dehydrogenase (aldehyde dismutase) of Paraburkholderia caribensis (protein code gi 1016073698, accession no. WP_062918235), which is a putative enzyme identified by complete genome sequencing (Table ). The two unique peptides from B43 overlapped with two of the seven unique peptides of A43 (data not shown). This result suggested that B43 also contained a formaldehyde dismutase. The possible formaldehyde dismutase of A43 may be the same as that of B43 if it were eluted continually between fraction numbers 71 and 93 during hydrophobic interaction chromatography. Another possibility is that the possible formaldehyde dismutase of A43 is distinct from that of B43. In B60, B43, and B36, the methanol dehydrogenase (large subunit), the isocitrate dehydrogenase, and the glyceraldehyde-3-phosphate dehydrogenase were detected (Table ). Since it is believed that these enzymes do not catalyze the oxidation of formaldehyde at least without the addition of an electron acceptor, it appears that the formaldehyde dismutase activity in B-fraction of the hydrophobic interaction chromatography as described above depended on only the formaldehyde dismutase.
Figure 2. SDS-PAGE of the fractions from hydrophobic interaction chromatography with formaldehyde dismutase activity. Lanes A and B, the representatives of A-fraction and B-fraction, respectively, from Figure 1. Lane M, protein markers. Arrows indicate the molecular weights of bands from the two fractions. Experimental conditions are specified in Materials and methods.
Table 2. NanoLC–MS/MS analysis of the gel bands from SDS-PAGE of the fractions from hydrophobic interaction chromatography with formaldehyde disumutase activity.
Cloning of a formaldehyde dismutase gene from Methylobacterium sp. FD1
We cloned the FD1 formaldehyde dismutase gene by PCR based on the peptide information of A43 in this study. First, the middle region (0.9 kb) of the formaldehyde dismutase gene was amplified from FD1 genomic DNA. Next, the upstream (0.34 kb) and downstream (1.3 kb) regions that link with the middle region were amplified by TAIL-PCR from FD1 genomic DNA. From the DNA sequence (1437 bp) that was overlapped by the three amplified fragments, the open reading frame of the possible formaldehyde dismutase gene of FD1 was estimated to be 1203 bp (accession number LC269930). Although the ATG start codon of the gene is speculative, it is likely to be the start codon because a TAA stop codon resides 30 bp upstream of this ATG, there is no start codon between the TAA and the ATG, and a possible Shine–Dalgarno sequence was found 12–7 bp upstream of the ATG.
We next sought to confirm that the protein expressed from the possible formaldehyde dismutase gene of FD1 had formaldehyde dismutase activity. A full-length possible FD1 formaldehyde dismutase gene was amplified by PCR from FD1 genomic DNA and ligated under a rhaPBAD promoter of pRham™ vector (pRham-FDM) in E. coli. The resting cells of the recombinant E. coli completely degraded 0.33 M formaldehyde in 30 min, though most cells were dead after 10 min, and produced formic acid and methanol that were approximately molar equivalents of one-half of the degraded formaldehyde (Figure ). E. coli carrying pRham (no insert) did not degrade formaldehyde. The SDS-PAGE analysis showed a specific band around 41 kDa in the cell-free protein extract from the recombinant E. coli with the rhamnose induction (Figure ). This value was close to the molecular weight (42.877.32) calculated from the deduced formaldehyde dismutase of FD1. These results showed that the gene cloned from FD1 coded formaldehyde dismutase. The isoelectoric point (pI) of this enzyme was calculated as 6.56. To our knowledge, there are no reports of the cloning of a formaldehyde dismutase gene except for that of P. putida [Citation5,6,8].
Figure 3. Degradation of formaldehyde and production of formic acid and methanol by resting cells of E. coli transformed with the FD1 formaldehyde dismutase gene and decrease in viable cells of the recombinant E. coli. The reaction mixture contained 1 OD-unit/mL of the cells. The solid lines, the recombinant E. coli with pRham-FDM; the dashed line, the recombinant E. coli with pRham (no insert); Closed circles, formaldehyde; closed triangles, formic acid; closed squares, methanol; open circles, viable cells of the recombinant E. coli in the buffer with 0.33 M formaldehyde; open triangles viable cells of the recombinant E. coli in the buffer without formaldehyde. Experimental conditions are specified in Materials and methods. All experiments were performed in triplicates, and the mean ± standard deviation is shown.
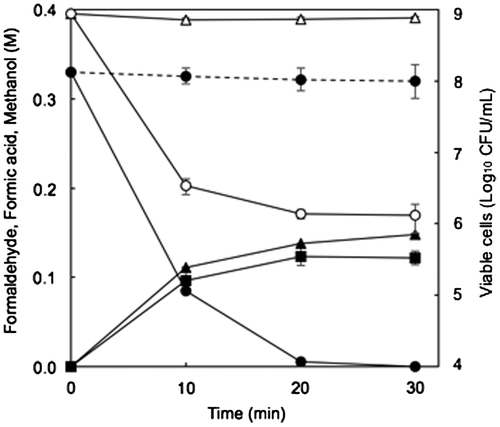
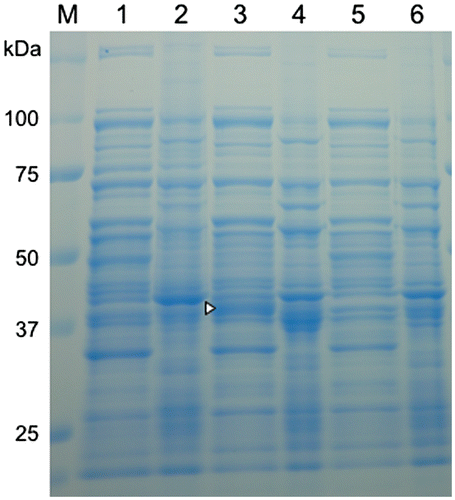
Figure 4. SDS-PAGE of protein extracts and precipitate fractions of E. coli transformed with the formaldehyde dismutase gene of FD1. For all samples, 10 μg of total protein were loaded. Lanes 1–4, the recombinant E. coli with pRham-FDM; lanes 5 and 6, the recombinant E. coli with pRham (no insert); lanes 1, 3, and 5, cell-free protein extracts; lanes 2, 4, and 6, precipitate fractions; lanes 1 and 2, no rhamnose induction; lanes 3, 4, 5, and 6, rhamnose induction. A triangle indicates a specific band. Experimental conditions are specified in Materials and methods.
Homology search for formaldehyde dismutase of Methylobacterium sp. FD1
As a result of the homology search for the amino acid sequence deduced from the formaldehyde dismutase gene of FD1, more than 100 aldehyde dismutases (that were identified as homologs of glutathione-independent formaldehyde dehydrogenase) or glutathione-independent formaldehyde dehydrogenases with identities of 93–60% were detected (data not shown). All of these enzymes, except for the formaldehyde dismutase of P. putida F61 [Citation5], were predicted by complete genome sequence analysis. The alignment results for 10 amino acid sequences, including those of the formaldehyde dismutase of FD1, the formaldehyde dismutase of P. putida F61 [Citation5], and the glutathione-independent formaldehyde dehydrogenase of P. putida C83 [Citation8], which have been purified and characterized, and six putative aldehyde dismutases and one putative glutathione-independent formaldehyde dehydrogenase, which are representative of enzymes from bacteria belonging to the different hierarchical classes detected by the homology search, are shown in Figure . Approximately one-third of the overall amino acid residues in these enzymes was conserved. The NAD(H)-binding residues, catalytic zinc-binding residues, and structural zinc-binding residues of the glutathione-independent formaldehyde dehydrogenase of P. putida C83 [Citation2] were found in these enzymes, including the formaldehyde dismutase of FD1, by BLAST search at the NCBI. The formaldehyde dismutase of FD1 that had been partially purified by hydrophobic interaction chromatography degraded formaldehyde without the addition of a coenzyme, such as NAD(H), as described above. Taken together, these data indicate that the formaldehyde dismutase of FD1 is likely to be a nicotinoprotein, like the NAD(H)-binding formaldehyde dismutase of P. putida [Citation2–5]. The alignment results suggested that the six putative aldehyde dismutases and one putative glutathione-independent formaldehyde dehydrogenase used for the alignment analysis may be formaldehyde dismutases, and that formaldehyde dismutase genes may have spread widely through bacteria. Methylobacterium sp. FD1 may have acquired the formaldehyde dismutase gene of another bacterium, such as Cupriavidus in class Betaproteobacteria, by horizontal gene transfer.
Figure 5 Amino acid alignment of the formaldehyde dismutase of FD1 with (putative) enzymes related to formaldehyde dismutase. The sequences used for the alignment are as follows: MspFD1-FDM, formaldehyde dismutase of Methylobacterium sp. FD1 (accession No. LS269930) in class Alphaproteobacteria; CspSK3-ADM, putative aldehyde dismutase of Cupriavidus sp. SK-3 (WP_051648669) in class Betaproteobacteria; Tbo-ADM, putative aldehyde dismutase of T. bouteillei (WP_038092476) in class Cyanobacteria; MspGXS13-ADM, putative aldehyde dismutase of Methylobacterium sp. GXS13 (WP_058191240) in class Alphaproteobacteria; Pbo-FDH, putative glutathione-independent formaldehyde dehydrogenase of P. borealis (WP_076349806) in class Planctomycetia; Rsp-ADM, putative aldehyde dismutase of Rhodococcus spp. (WP_003943355) in class Actinobacteria; SspTu6071-ADM, putative aldehyde dismutase of Streptomyces sp. Tu6071 (WP_043257889) in class Actinobacteria; Fsp-ADM, putative aldehyde dismutase of Flavobacteriaceae (WP_027374817) in class Flavobacteriia; PpuF61-FDM, formaldehyde dismutase of P. putida F61 (L25862) of Gammaproteobacteria; PpuC83-FDH, glutathione-independent formaldehyde dehydrogenase of P. putida C83 (D21201) of Gammaproteobacteria. Identical residues in all of the (putative) enzymes are indicated by black highlight. Asterisks, the NAD(H)-binding residues; open circles, catalytic zinc-binding residues; closed circles, structural zinc-binding residues of the glutathione-independent formaldehyde dehydrogenase of P. putida C83 [Citation2].
![Figure 5 Amino acid alignment of the formaldehyde dismutase of FD1 with (putative) enzymes related to formaldehyde dismutase. The sequences used for the alignment are as follows: MspFD1-FDM, formaldehyde dismutase of Methylobacterium sp. FD1 (accession No. LS269930) in class Alphaproteobacteria; CspSK3-ADM, putative aldehyde dismutase of Cupriavidus sp. SK-3 (WP_051648669) in class Betaproteobacteria; Tbo-ADM, putative aldehyde dismutase of T. bouteillei (WP_038092476) in class Cyanobacteria; MspGXS13-ADM, putative aldehyde dismutase of Methylobacterium sp. GXS13 (WP_058191240) in class Alphaproteobacteria; Pbo-FDH, putative glutathione-independent formaldehyde dehydrogenase of P. borealis (WP_076349806) in class Planctomycetia; Rsp-ADM, putative aldehyde dismutase of Rhodococcus spp. (WP_003943355) in class Actinobacteria; SspTu6071-ADM, putative aldehyde dismutase of Streptomyces sp. Tu6071 (WP_043257889) in class Actinobacteria; Fsp-ADM, putative aldehyde dismutase of Flavobacteriaceae (WP_027374817) in class Flavobacteriia; PpuF61-FDM, formaldehyde dismutase of P. putida F61 (L25862) of Gammaproteobacteria; PpuC83-FDH, glutathione-independent formaldehyde dehydrogenase of P. putida C83 (D21201) of Gammaproteobacteria. Identical residues in all of the (putative) enzymes are indicated by black highlight. Asterisks, the NAD(H)-binding residues; open circles, catalytic zinc-binding residues; closed circles, structural zinc-binding residues of the glutathione-independent formaldehyde dehydrogenase of P. putida C83 [Citation2].](/cms/asset/f27c29d7-f5a1-4778-abd8-69f4d2f60cc0/tbbb_a_1397497_f0005_b.gif)
Degradation of high concentrations of formaldehyde by resting and lyophilized cells of recombinant E. coli
Resting cells: One OD-unit/mL of the cells degraded 98% of 0.67 M formaldehyde after 1.5 h (Figure ). One OD-unit/mL and 5 OD-units/mL of the cells degraded 76 and 95% of 1.3 M formaldehyde after 1.5 h, respectively. The formaldehyde degradation activity of the recombinant E. coli cells with pRham-FDM was estimated as approximately 24 units/OD-unit and it was higher than that of FD1 cells (approximately 2 units/OD-unit). On the other hand, the doubling time of the recombinant E. coli and FD1 cells were 0.5 and 8.4 h in LB medium without formaldehyde, respectively. These results suggested that E. coli was suitable host for cell production with formaldehyde dismutase activity. The production of the formaldehyde dismutase of P. putida by recombinant E. coli have been reported [Citation5,6,8,11].
Figure 6. Degradation of high concentrations of formaldehyde by resting and lyophilized cells of E. coli transformed with the formaldehyde dismutase gene of FD1. (A) Resting cells. The reaction mixture contained 1 OD-unit/mL (circles) and 2 OD-units/mL (triangles) of the cells. (B) Lyophilized cells. The reaction mixture contained 1 OD-unit/mL (circles), 2 OD-units/mL (triangles), 5 OD-units/mL (squares), and 10 OD-units/mL (diamonds) of the cells. Experimental conditions are specified in Materials and methods. All experiments were performed in triplicates, and the mean ± standard deviation is shown.
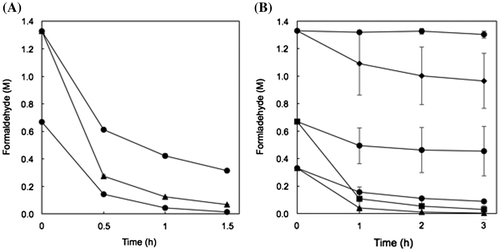
Lyophilized cells: One and 2 OD-units/mL of the cells that were already dead (data not shown) degraded 73 and 99% of 0.33 M formaldehyde after 3 h (Figure ). Five OD-units/mL of the cells degraded 96% of 0.67 M formaldehyde and 10 OD-Units/mL of the cells degraded 27% of 1.3 M formaldehyde after 3 h, although 1 OD-unit/mL of the cells did not degrade 0.67 and 1.33 M formaldehyde very much. It is thought that the reason for the lower degradation activity of the lyophilized cells than that of the resting cells is because the initial lyophilized cells were dead, but the details are unclear. To our knowledge, this is the first report of degradation of formaldehyde by lyophilized recombinant E. coli cells. The lack of a need for an expensive coenzyme is advantageous for the industrial use of the recombinant cells for degradation of formaldehyde. Therefore, resting and lyophilized E. coli cells transformed with the formaldehyde dismutase gene of FD1 are good candidates for the degradation (i.e. dismutation) of high concentrations of formaldehyde in its waste water. Further work such as purification and characterization of the formaldehyde dismutase of FD1 is needed to achieve the effective formaldehyde degradation.
Conclusion
In the present study, formaldehyde dismutase was partially purified and analyzed by nanoLC–MS/MS from Methylobacterium sp. FD1, and the formaldehyde dismutase gene (open reading frame, 1203 bp) was cloned by PCR based on the peptide sequence of the enzyme. NAD(H)-binding residues, catalytic zinc-binding residues, and structural zinc-binding residues were found in formaldehyde dismutase (401aa), as deduced from the formaldehyde dismutase gene of FD1. Resting cells of E. coli transformed with the formaldehyde dismutase gene of FD1 degraded high concentrations of formaldehyde and produced formic acid and methanol in approximately molar equivalents of one-half of the degraded formaldehyde. The lyophilized cells of the recombinant E. coli also degraded formaldehyde. The resting and lyophilized cells of E. coli transformed with the formaldehyde dismutase gene of FD1 are good candidates for agents to degrade high concentrations of formaldehyde in its waste water.
Authors’ contributions
HY designed and directed the project. YK performed the experiments in Figure . HY and YK performed the experiments in Figure . HY performed the experiments in Figures , , , and , analyzed the data, and wrote the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS KAKENHI) [grant number 26340065].
Acknowledgments
We thank Dr. Noboru Kishimoto, Dr. Yoshiharu Okuno and Mr. Seiji Imoto of National Institute of Technology, Wakayama College for helping for HPLC analysis and GC analysis.
References
- Yonemitsu H, Shiozaki E, Hitotsuda F, et al. Biodegradation of high concentrations of formaldehyde by lyophilized cells of Methylobacterium sp. FD1. Biosci Biotechnol Biochem. 2016;80:2264–2270.
- Tanaka N, Kusakabe Y, Ito K, et al. Crystal structure of formaldehyde dehydrogenase from Pseudomonas putida: the structural origin of the tightly bound cofactor in nicotinoprotein dehydrogenases. J Mol Biol. 2002;324:519–533.
- Kato N, Kobayashi H, Shimao M, et al. Properties of formaldehyde dismutation catalyzing enzyme of Pseudomonas putida F61. Agric Biol Chem. 1984;48:2017–2023.
- Kato N, Yamagami T, Shimao M, et al. Formaldehyde dismutase, a novel NAD-binding oxidoreductase from Pseudomonas putida F61. Eur J Biochem. 1986;156:59–64.
- Yanase H, Noda H, Aoki K, et al. Cloning, sequence analysis, and expression of the gene encoding formaldehyde dismutase from Pseudomonas putida F61. Biosci Biotechnol Biochem. 1995;59:197–202.
- Blaschke L, Wagner W, Werkmeister C, et al. Development of a simplified purification method for a novel formaldehyde dismutase variant from Pseudomonas putida J3. J Biotechnol. 2017;241:69–75.
- Ando M, Yoshimoto T, Ogushi S, et al. Formaldehyde dehydrogenase from Pseudomonas putida. J Biochem. 1979;85:1165–1172.
- Ito K, Takahashi M, Yoshimoto T, et al. Cloning and high-level expression of the glutathione-independent formaldehyde dehydrogenase gene from Pseudomonas putida. J Bacteriol. 1994;176:2483–2491.
- Haldimann A, Daniels LL, Wanner BL. Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J Bacteiol. 1998;180:1277–1286.
- Liu Y-G, Whittier RF. Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics. 1995;25:674–681.
- Yanase H, Moriya K, Mukai N, et al. Effects of GroESL coexpression on the folding of nicotinoprotein formaldehyde dismutase from pseudomonas putida F61. Biosci Biotechnol Biochem. 2002;66:85–91.
