Abstract
Coenzyme Q (CoQ) is essential for mitochondrial respiration and as a cofactor for sulfide quinone reductase. Schizosaccharomyces pombe produces a human-type CoQ10. Here, we analyzed CoQ in other fission yeast species. S. cryophilus and S. octosporus produce CoQ9. S. japonicus produces low levels of CoQ10, although all necessary genes for CoQ synthesis have been identified in its genome. We expressed three genes (dps1, dlp1, and ppt1) for CoQ synthesis from S. japonicus in the corresponding S. pombe mutants, and confirmed that they were functional. S. japonicus had very low levels of oxygen consumption and was essentially respiration defective, probably due to mitochondrial dysfunction. S. japonicus grows well on minimal medium during anaerobic culture, indicating that it acquires sufficient energy by fermentation. S. japonicus produces comparable levels of ethanol under both normal and elevated temperature (42 °C) conditions, at which S. pombe is not able to grow.
Schizosaccharomyces japonicus and Schizosaccharomyces pome are distinct in mitochondrial-related functions.
Schizosaccharomyces fission yeast species are believed to have diverged from Saccharomyces budding yeast species about a billion years ago. Fission yeasts are named based on their binary fission cell division pattern, in contrast to the cellular budding division pattern in Saccharomyces. Four fission yeast species are currently known, and all belong to the genus Schizosaccharomyces, including S. pombe, S. japonicus [Citation1], S. cryophilus [Citation2], and S. octosporus [Citation3]. S. pombe has been extensively studied in genetic, molecular biological, biochemical, and cytological analyses [Citation4], but studies of the other three species are limited. The S. pombe whole genome was completely sequenced by 2002 [Citation5] and the whole genomes of the other three species were determined in 2011 [Citation6]. Genomic differences among the four Schizosaccharomyces species were determined; 3,924 genes are common among the four species, whereas 133–401 genes are different.
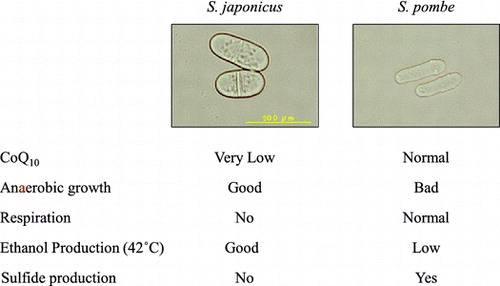
S. japonicus is a dimorphic yeast, which can transit from unicellular yeast to long filamentous hyphae, and form ascospores with eight spores when starved [Citation1]. S. japonicus was isolated in 1928 in Japan, and is currently undergoing re-evaluation because of its unique properties [Citation7]. Nuclear organization and division have been investigated in S. japonicus [Citation1], but physiological studies of this yeast are limited. A prominent characteristic of S. japonicus is that it does not respire, and instead grows via fermentation. It was reported that S. japonicus does not produce Coenzyme Q (CoQ) [Citation8], despite its essential role in respiration and oxidative ATP synthesis in mitochondria. CoQ synthesis in eukaryotes has been studied primarily in the Saccharomyces cerevisiae budding yeast [Citation9–11] and the S. pombe fission yeast, and these knowledge extended to higher eukaryotes [Citation12–14], but has not been studied in S. japonicus. As CoQ is synthesized in mitochondria, it is interesting to know how S. japonicus adapted to deficiency of its synthesis, which causes respiration deficiency.
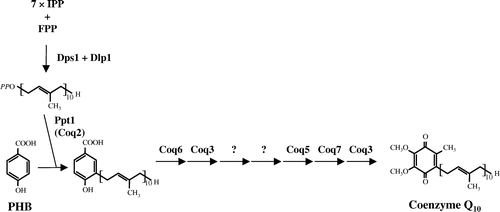
CoQ contains a quinone frame and isoprenoid side chain, with variable isoprene units in each organism. S. cerevisiae produces CoQ6, whereas S. pombe and Homo sapiens produce CoQ10 [Citation10,12,15]. CoQ isoprenoid side chains are synthesized by the homomeric form of Coq1 (hexaprenyl diphosphate synthase) in S. cerevisiae, and by the heterotetrameric form of Dps1 and Dlp1 (decaprenyl diphosphate synthase) in S. pombe [Citation14]. The type of CoQ such as CoQ6 in S. cerevisiae and CoQ10 in S. pombe is determined by the supplied prenyl diphosphate synthesized by the species-specific polyprenyl diphosphate synthase [Citation16,17]. After synthesis, the isoprenoid is transferred to p-hydroxy benzoate (PHB) by Coq2 (Ppt1) (PHB-polyprenyl diphosphate transferase) [Citation18,19]. Prenylated PHB undergoes the following modifications: hydroxylation by Coq6 and Coq7, O-methylation by Coq3, C-methylation by Coq5, and decarboxylation by an unknown protein (Figure ) [Citation13]. Almost all CoQ synthetic genes in humans and Arabidopsis thaliana can function to complement each of the corresponding S. pombe gene deletion mutants [Citation20]. Biotechnology approaches have successfully enhanced CoQ10 biosynthesis in S. pombe fission yeast [Citation21,22]. Therefore, an analysis of CoQ biosynthesis in other fission yeast species may provide insights for the utilization of fission yeast for commercial production of CoQ10.
Figure 1. Proposed coenzyme Q (CoQ) biosynthetic pathway in S. pombe. The biosynthetic pathway that converts PHB into CoQ consists of eight steps in S. pombe. Decaprenyl diphosphate which is synthesized by decaprenyl diphosphate synthase (Dps1 + Dlp1) is transferred to PHB by PHB-decaprenyl diphosphate transferase (Ppt1 (Coq2)), and then seven modifications of the aromatic ring are performed in CoQ biosynthesis.
In this study, we investigated CoQ synthesis in S. japonicus, genes involved in CoQ synthesis in S. pombe, and yeast phenotypes associated with respiration and ethanol production. We show that evolutionally unique properties of S. japonicus lost major mitochondrial function and enforced fermentation for energy acquirement.
Materials and methods
Yeast strains and media
The genotypes of all yeast strains used in this study are listed in Table . S. pombe and S. japonicus strains were grown in YES medium (0.5% yeast extract, 3% glucose, and 225 mg/L each of adenine, leucine, uracil, histidine, and lysine hydrochloride), YPD medium (1% yeast extract, 2% glucose, and 2% polypeptone), or EMM synthetic medium containing nutritional supplements when necessary [Citation23]. Yeast cells were transformed using either lithium acetate [Citation24] or electroporation [Citation25]. General genetic methods used for S. pombe have been described previously [Citation26]. The thiamine-repressible nmt1 promoter was repressed by adding 5 μg/ml thiamine to EMM medium.
Table 1. Strains used in this study.
DNA manipulation
Cloning, restriction enzyme analysis, and plasmid DNA preparation were performed essentially as described previously [Citation27]. Oligonucleotides used in this study are listed in Table S1. Escherichia coli strain DH5α was used for plasmid construction and propagation. DNA sequences were determined using the dideoxynucleotide chain-termination method and the ABI377 DNA sequencer.
Plasmid construction
The plasmids used in this study are listed in Table , and the primers used for plasmid construction are listed in Table S1. The pREP41-Sjppt1 plasmid was constructed by amplifying a fragment using the Sjppt1(ORF)-SalI-F and Sjppt1-BamHI-R primers, and inserting the amplified product into the SalI and BamHI sites of pREP41. The pREP1-Sjdps1, pREP41-Sjdps1, and pREP2-Sjdps1 plasmids were constructed by amplifying a fragment using the Sjdps1-SalI-F and Sjdps1-BamHI-R primers, and inserting the amplified product into the SalI and BamHI sites of pREP1, pREP41, and pREP2, respectively. The pREP1-Sjdlp1 and pREP2-Sjdlp1 plasmids were constructed by amplifying a fragment using the Sjdlp1-SalI-F and Sjdlp1-BamHI-R primers, and inserting the amplified product into the SalI and BamHI sites of pREP1 and pREP2, respectively. The pREP41-dps1 and pREP2-dps1 plasmids were constructed inserting the dps1 gene which was cut from pREP1-cloning plasmid by restriction enzymes into the same sites of pREP41 and pREP2, respectively. The pSJU11-Spppt1–15 plasmid was constructed by amplifying fragments using the Sjnmt1–897-F and Sjnmt1–24-R primers for Sjnmt1 promoter, and Spppt1-Sjnmt1–24-F-New and Spppt1-BamHI-R primers for Spppt1 coding gene. Amplified fragments were fused by PCR reaction, and the product was cloned into the KpnI and BamHI sites of pSJU11.
Table 2. Plasmids used in this study.
Spot assay
Cells were grown on YES plates for 3 days at 30 °C, and then resuspended in water to a density of 2 × 10Citation6 cells/ml. Cell suspensions were serially diluted (1:10), spotted onto YES or EMMU plates, and incubated for 3–5 days at 30 °C. Plates were placed in a sealed chamber under anaerobic conditions with AnaeroPack Kenki (Mitsubishi Gas Chemical Co., Inc., Tokyo), and incubated for 2 days at 30 °C.
CoQ extraction and measurement
CoQ was extracted as described previously [Citation28]. The CoQ crude extract was analyzed by normal-phase thin-layer chromatography (TLC) with authentic CoQ6 or CoQ10 standards. Normal-phase TLC was conducted on a Kieselgel 60 F254 plate and developed with benzene. The plate was viewed under UV illumination, the CoQ band was collected, and the sample was extracted with hexane/isopropanol (1:1, v/v). Samples were dried and solubilized in ethanol. Purified CoQ was subjected to high-performance liquid chromatography (HPLC) with ethanol as the solvent.
Liquid chromatography–mass spectrometry (LC-MS) analysis
The CoQ sample was extracted for liquid chromatography–mass spectrometry (LC-MS) analysis as described above. Samples were resuspended in 80 μl of methanol:2-propanol (4:1) solution and filtered with YMC Duo-Filter XQ DUO 04 (pore size, 0.2 μm), and 8 μl of sample was used for analysis. LC-MS data were obtained using a MassLynx system (Waters) coupled to a Xevo-TQS mass spectrometer (Waters). LC separation was performed on an ACQUITY UPLC BEH C18 column (2.1 × 50 mm, 1.7 μm particle size; Waters). The mobile phase was methanol:2-propanol (4:1) solution (buffer A) and methanol:2-propanol (4:1) solution containing 5 mM ammonium formate (buffer B). Chromatographic conditions were 98% buffer A and 2% buffer B. The flow rate was 0.5 ml/min. Matrix-assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF MS) (SYNAPT G2-S; Waters) was performed to determine the precise molecular masses of compounds.
Sulfide measurement
Sulfide content was determined quantitatively using the methylene blue method as described previously [Citation14]. Briefly, S. pombe and S. japonicus cells were grown in YES medium (50 ml) until the late log phase. Then, cells were collected and disrupted by glass beads, and cell extracts were resuspended in 0.1 ml of 0.1% dimethylphenylenediamine (in 5.5 N HCl) and 0.1 ml of 23 mM FeCl3 (in 1.2 N HCl). The samples were incubated at 37 °C for 5 min, and the sample absorbance at 670 nm was determined using a blank containing only the reagents.
Oxygen consumption
Oxygen consumption was measured in the medium where the tested strains were grown using the YSI model 53 oxygen monitor (YSI, Inc.). Cells were cultured until log phase in YES medium at 30 °C. Cells were collected by centrifugation, washed in MilliQ water, and resuspended in water to a density of 1 × 10Citation9 cells/ml. Then, 3 ml of air-saturated culture was used to calculate the rate of oxygen consumption.
Ethanol measurement
Ethanol production by the tested strains was measured using a refractive index detector (Shimazu HPLC LC6AD) equipped with an ULTRON PS80-H column. Ethanol was quantified by differential refractive index with glycerol as a standard.
Results
Respiration is deficient in Schizosaccharomyces japonicus
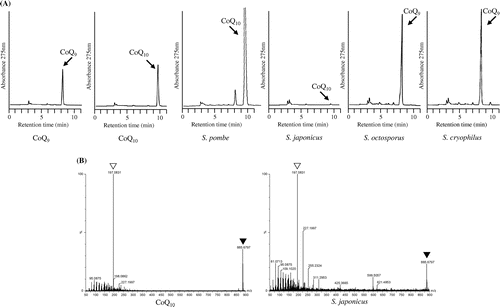
We measured CoQ species and their contents in four fission yeast species: S. pombe, S. cryophilus, S. octosporus, and S. japonicus. S. pombe produces CoQ10 [Citation13,14]. We confirmed an earlier report that S. octosporus produces CoQ9. The type of CoQ produced in S. cryophilus was unknown; we identified CoQ9, similar as in S. octosporus. A previous study reported that S. japonicus does not produce detectable CoQ [Citation8], but we detected a very small amount of CoQ10 using HPLC analysis (Figure (A)). The CoQ10 content was approximately 0.167 μg/1 × 109 cells or 0.3 μg/100 ml of culture, which is approximately 220 times lower than the CoQ10 content in S. pombe grown in YES medium (37 μg/1 × 109 cells or 69.5 μg/100 ml of culture). We subjected the sample to MS analysis (Figure (B)). A peak appearing at 885.6797 m/z [M + Na]+ corresponded with CoQ10, and a peak at 197.0831 m/z [M]+ by MS/MS corresponded with tropylium ion [M]+ [Citation9]. These results verified that this product is CoQ10.
Figure 2. CoQ contents in four fission yeasts. (A) HPLC analyses of CoQs from S. pombe PR110, S. japonicus NIG5091, S. octosporus yFS286, and S. cryophilus OY26 with CoQ9 and CoQ10 standards. (B) MS analysis of CoQ produced in S. japonicus. Open triangle marks peak at 197.0831 m/z; closed triangle marks peak at 885.6797 m/z. It is identical to standard CoQ10.
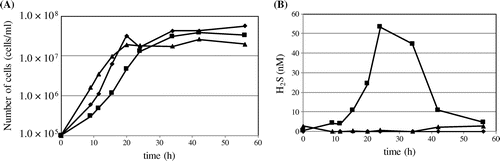
Because the amount of CoQ was very low in S. japonicus, we measured the respiration capacity. We tested the growth of S. japonicus on non-fermentable carbon sources. S. japonicus and the S. pombe CoQ-deficient mutant (∆ppt1) could not grow on 2% glycerol + 1% ethanol as carbon sources (Figure (A)). Next, we measured oxygen consumption of S. japonicus and compared it with that of S. pombe wild type and ppt1 mutants (Figure (B)). S. japonicus did not consume oxygen, which was similar to the S. pombe respiration-deficient mutant. These combined results suggest that S. japonicus can grow well under anaerobic conditions. We measured the growth of S. japonicus under oxygen-depleted conditions, and compared it with that of S. pombe wild type and CoQ-deficient mutants (Figure ). Under anaerobic conditions, S. japonicus grew much faster than S. pombe wild type and CoQ-deficient mutants. There was no difference in S. japonicus growth under aerobic and anaerobic conditions, whereas S. pombe and S. cerevisiae grew much faster under aerobic conditions, and growth of the S. pombe CoQ-deficient mutants was slow.
Figure 3. Respiration deficiency of S. japonicus. (A) S. pombe wild type (PR110), S. pombe ∆ppt1, and S. japonicus wild type NIG5091 were grown on YES medium containing 3% glucose for 5 days or 2% glycerol + 1% ethanol for 7 days at 30 °C. (B) Oxygen consumption was monitored in S. pombe wild type PR110 (diamond), S. pombe ∆ppt1 (square), and S. japonicus wild type NIG5091 (triangle).
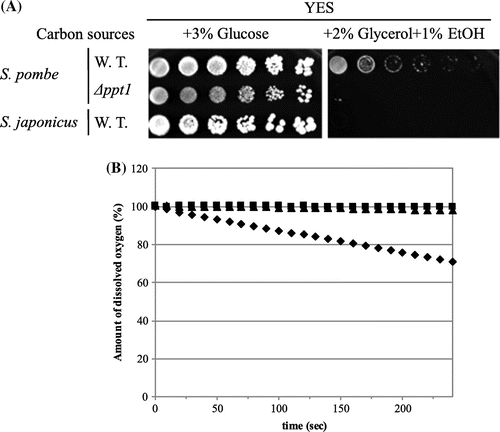
Figure 4. Growth under anaerobic conditions. S. japonicus (NIG2028, NIG5091), S. pombe (WT (PR110) and ∆ppt1), and S. cerevisiae (WT (W303–1A) and ∆coq2) [Citation38] strains were grown, serially diluted, and spotted on YES and YPAD for 2 days under aerobic and anaerobic conditions.
![Figure 4. Growth under anaerobic conditions. S. japonicus (NIG2028, NIG5091), S. pombe (WT (PR110) and ∆ppt1), and S. cerevisiae (WT (W303–1A) and ∆coq2) [Citation38] strains were grown, serially diluted, and spotted on YES and YPAD for 2 days under aerobic and anaerobic conditions.](/cms/asset/88b35a4c-6d5b-4807-89bb-71c6b2a0623b/tbbb_a_1401914_f0004_b.gif)
Sensitivity to oxidative stress
S. pombe coq deletion mutants are sensitive to H2O2 [Citation29]. To determine the S. japonicus oxidative stress sensitivity, we determined the sensitivity to H2O2 and paraquat (PQ). S. japonicus was sensitive to both H2O2 and PQ (Figure ). Wild type S. pombe does not display oxidative stress sensitivity. S. japonicus has much greater oxidative stress sensitivity than S. pombe ppt1 (coq2) mutants.
Sulfide production
S. pombe coq mutants produce higher sulfide levels than the wild type due to defective sulfide quinone reductase activity in mitochondria [Citation30–32]. As S. japonicus produces very little CoQ10, we assessed the amount of sulfide produced in S. japonicus. S. japonicus did not produce sulfide even though it produces almost no CoQ (Figure (B)). These combined results indicate that the metabolic regulation of sulfide and CoQ in mitochondria of S. japonicus differs from that in S. pombe.
Expression of CoQ biosynthetic genes in S. pombe
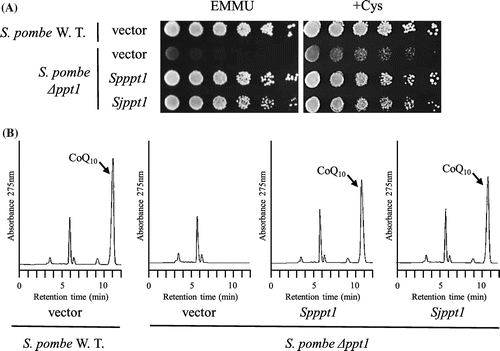
We investigated possible reasons for low CoQ10 levels in S. japonicus by performing complementation assays of CoQ biosynthetic genes in S. pombe. We tested three genes involved in early steps of CoQ biosynthesis: ppt1, dps1, and dlp1. These genes were isolated from S. japonicus by searching databases using S. pombe homolog sequences for Ppt1, Dps1, and Dlp1 [National Center for Biotechnology Information (NCBI) BLAST program]. Homologous proteins [SJAG_06603 (named SjPpt1), SJAG_04568 (named SjDps1), and SJAG_05776 (named SjDlp1)] were identified, and amino acid sequence alignments of these proteins are shown in Figs. S1, S2, and S3. Ppt1 (Coq2) condenses polyprenyl diphosphate with PHB [Citation19]. SjPpt1 was identified, but the annotation stated that the first methionine was absent. When we carefully searched the S. japonicus genome data, the ATG codon was found in the 5′ upstream region of SJAG_06603 and no other ATG codon was found around there. We were able to find the real open reading frame (ORF) of SJAG_06603 in the S. japonicus NIG5091 genome. Then, we tested Sjppt1 expression in the S. pombe ∆ppt1 strain. The delayed growth of S. pombe ∆ppt1 in minimal medium was complemented by the S. japonicus Sjppt1 gene (Figure (A)). SjPpt1 functioned well and restored CoQ10 production in S. pombe ∆ppt1 (Figure (B)). We also expressed S. pombe ppt1 in S. japonicus, but did not observe any significant increase in CoQ10 (Figure S4). Furthermore, we observed that addition of PHB increases the CoQ10 levels in S. japonicus (Figure S5) and mitochondria show weak staining with Mitotracker (data not shown). We believe that the reason for the lack of CoQ synthesis is not due to Ppt1 function.
Figure 7. Expression of S. japonicus ppt1 in S. pombe ∆ppt1 strain. S. pombe wild type (PR110) harboring pREP41 and S. pombe ∆ppt1 harboring pREP41, pSLF272LGFP-Ppt1, or pREP41-Sjppt1 were grown in minimal medium with or without cysteine for 4 days at 30 °C (A). Production of CoQ10 was measured by HPLC (B). CoQ6 was used as standard.
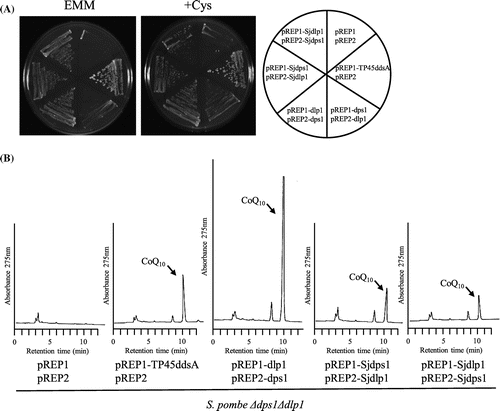
S. japonicus Sjdps1 and Sjdlp1 are homologous to dps1 and dlp1, respectively, which are highly likely to encode prenyl diphosphate synthases [Citation14,28,33]. We tested the functionality of S. japonicus dps1 and dlp1 in the corresponding S. pombe deletion mutants. When Sjdps1 was expressed in the S. pombe dps1 deletion mutant, it restored growth in minimal medium (Figure (A)) but produced little CoQ10 (Figure (B)). When Sjdlp1 was expressed in the S. pombe dlp1 deletion mutant, it restored growth in minimal medium (Figure (A)) and produced normal levels of CoQ10 (Figure (B)). When Sjdps1 and Sjdlp1 were expressed in the S. japonicus dps1 dlp1 double mutant, they restored growth in minimal medium (Figure (A)) and produced equivalent CoQ10 levels to those produced by the homomer ddsA gene fused to the mitochondrial targeting sequence (Figure (B)). We swapped the cloning vector of Sjdps1 and Sjdlp1, but this did not affect growth or CoQ10 production. These combined results indicate that Sjppt1, Sjdps1, and Sjdlp1 are functional in S. pombe, suggesting that S. japonicus possesses functional genes.
Figure 8. Expression of S. japonicus dps1 in S. pombe ∆dps1 strain. S. japonicus dps1 was expressed in S. pombe ∆dps1 strain (LJ1030). Cells were grown on minimal medium with or without cysteine for 6 days at 30 °C (a), and synthesis of CoQ10 was measured by HPLC (B). Vector: LJ1030/pREP41; Spdps1: LJ1030/pREP41-dps1; Sjdps1–1 and Sjdps1–2: LJ1030/pREP41-Sjdps1–1 or pREP41-Sjdps1–2 (these plasmids were constructed independently, but used the same structure).
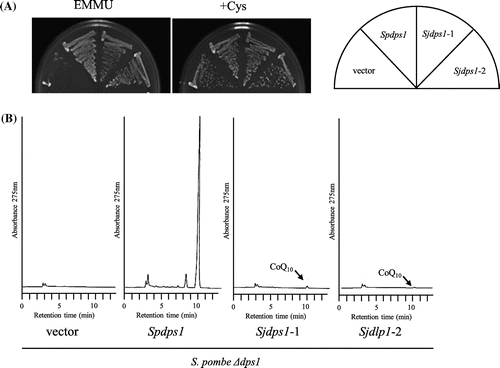
Figure 9. Expression of S. japonicus dlp1 in S. pombe ∆dlp1 strain. S. japonicus dlp1 was expressed in S. pombe ∆dlp1 strain (RM19). Cells were grown on minimal medium with or without cysteine for 5 days at 30 °C (A), and synthesis of CoQ10 was measured by HPLC (B). Vector: RM19/pREP1; Spdlp1: RM19/pREP1-dlp1; Sjdlp1–1 or Sjdlp1–2: RM19/pREP1-Sjdlp1–1 or pREP1-Sjdlp1–2 (these plasmids were constructed independently, but used the same structure).
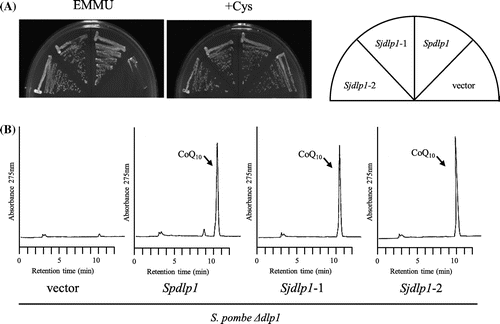
Figure 10. Expression of S. japonicus dlp1 and dps1 in the S. pombe ∆dlp1∆dps1 double mutant. S. japonicus dlp1 and dps1 were expressed in the S. pombe ∆dps1∆dlp1 double deletion strain (LA1). Cells were grown on the minimum medium with or without cysteine for 5 days at 30 °C (A), and synthesis of CoQ10 was measured (B).
Ethanol production by S. japonicus
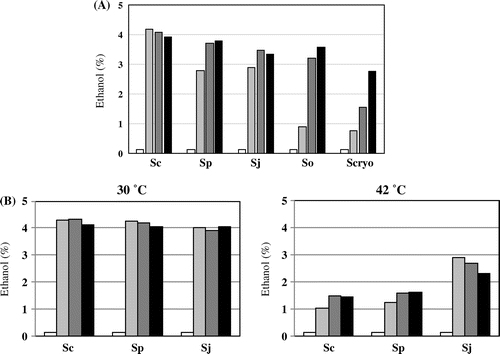
S. japonicus lacks respiration and grows by fermentation. Therefore, we expect that it might produce higher ethanol levels during fermentation. A previous study reported ethanol production by S. pombe [Citation34]. We measured the ethanol produced by the other three fission yeasts, S. japonicus, S. octosporus, and S. cryophilus (Figure ). S. japonicus produces comparable ethanol levels to S. pombe, whereas S. octosporus and S. cryophilus did not produce ethanol as efficiently as S. japonicus and S. pombe (Figure (A)). S. japonicus grew at 42 °C (Fig. S6); therefore, we measured ethanol production at 42 °C. At higher temperature, ethanol production was not as efficient as at 30 °C but significant ethanol was produced at 42 °C (Figure (B)). These results indicate that S. japonicus is potentially useful for ethanol production, especially at higher temperatures.
Figure 11. Ethanol production. (A) The amount of ethanol (v/v) produced in S. cerevisiae kyokai No. 9 (Sc), S. pombe L972 (Sp), S. japonicus NIG2028 (Sj), S. octosporus yFS286 (So), and S. cryophilus OY26 (Scryo) was measured by HPLC at 0 (white bar), 24 (light gray bar), 48 (dark gray bar), and 72 (black bar) hours. Cells were grown at 25 °C in YPD (10% glucose). (B) The amount of ethanol produced in S. cerevisiae kyokai No. 9 (Sc), S. pombe L972 (Sp), and S. japonicus NIG2028 (Sj) was measured by HPLC at 0 (white bar), 24 (light gray bar), 48 (dark gray bar), and 72 (black bar) hours. Cells were grown either at 30 or 42 °C in YPD (10% glucose).
Discussion
In this study, we analyzed the physiological properties of the hyphal-forming fission yeast S. japonicus. We observed that S. japonicus did not respire, and it grew well under anaerobic conditions [Citation35]. We found that S. japonicus produces very low levels of CoQ10, is sensitive to oxidative stress, does not produce hydrogen sulfide as in S. pombe CoQ-deficient mutants [Citation30], and produces ethanol under higher temperatures (42 °C). S. japonicus is quite different from S. pombe in its mitochondrial dependency, even though these two species are within the same genus. S. japonicus was first isolated from a strawberry field in Kyushu, Japan. The reason why S. japonicus lacks respiration is unknown. We also isolated a natural S. japonicus species (S. japonicus Kinzaki in Matsue City). This strain also produced only a low level of CoQ10 and had defective respiration (data not shown). At least two other strains of S. japonicus have been isolated from natural environments in Nagano and Hirosaki, Japan. These strains also produced only a low level of CoQ10 and had deficient respiration (data not shown). At least four independently isolated strains display the same properties, so it is unlikely that the phenotypes we observed in this study are specific to certain strains. We measured very low levels of CoQ of S. japonicus, although a previous study reported that CoQ was not detected in S. japonicus [Citation8]. The low CoQ levels may cause respiration deficiency, but this is not conclusive. Low CoQ levels may be a consequence of mitochondrial dysfunction, but not a reason for respiration deficiency. Mitochondrial dysfunction in S. japonicus probably affects the production of hydrogen sulfide, which is synthesized in mitochondria.
We tried to determine why S. japonicus produces very little CoQ10 by analyzing the CoQ biosynthetic genes in the whole-genome sequence of S. japonicus [Citation6]. All genes [dps1, dlp1, ppt1 (coq2)-coq9] involved in CoQ synthesis were identified in the whole-genome data (Table ). We performed complementation analyses of Sjdps1, Sjdlp1, and Sjppt1 in the corresponding S. pombe mutants dps1, dlp1, and ppt1. The results show that the S. japonicus genes are functional and complement the S. pombe strains to produce CoQ10, which is consistent with the finding that S. japonicus naturally produces CoQ10 despite its level being very low. SjDps1 and SjDlp1 function as decaprenyl diphosphate synthases, similar as in S. pombe and H. sapiens [Citation14,15]. All coq genes, dps1, and dlp1 were confirmed by RNA seq analysis [Citation6], the expression levels in S. japonicus were within normal ranges, and the alignment of all Coq proteins was well-conserved. Although we did not test every gene related to CoQ biosynthesis, we think it unlikely that very low CoQ levels in S. japonicus are due to lack of specific CoQ genes. We also observed that addition of PHB increases the CoQ10 levels in S. japonicus (Figure S5), which suggests that the whole enzymatic reaction is not disrupted. CoQ synthetic enzymes are active, but have weak activity. The low level of CoQ synthesis results from mitochondrial incompleteness. Mitochondrial DNA is present [Citation36,37], and mitochondria show weak staining with Mitotracker (data not shown). Further analysis of mitochondrial function will be necessary to determine the reason for low CoQ10 synthesis in S. japonicus.
Table 3. CoQ biosynthetic genes in four fission yeasts.
S. japonicus is a unique yeast in that it evolved limited respiratory function. It grows much faster than S. pombe under anaerobic conditions (fermentation). We found that S. japonicus produces more ethanol at higher temperatures than the other three fission yeasts. S. japonicus and S. pombe produce comparable ethanol levels at 30 and 37 °C but S. japonicus has much more efficient ethanol production at 42 °C. Therefore, this yeast has great potential for ethanol production at higher temperatures or during fermentation to make beer or sake. S. japonicus smells better than S. pombe because it lacks hydrogen sulfide synthesis, which is a benefit for the production of alcoholic beverages.
Author contributions
K.T. S.M. and Y.T. performed the experiments and analyzed the data; M.K. and T. K. designed the experiments and wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Ministry of Education, Culture, Sports, Science, and Technology of Japan [grant number 15K07360]; Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry, Japan [grant number 27012A].
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/09168451.2017.1401914.
Fig-suppl_v8.pdf
Download PDF (2.1 MB)Acknowledgments
This work was technically supported by H. Akai and S. Matsumoto. We thank Drs. T. Tonouchi (Hirosaki University) and K. Nozaki (Shinshu University) for allowing us to use naturally isolated S. japonicus. We also thank Drs. T. Nakagawa and Y. Matsuo for their scientific advice. The S. japonicus strains and pSJU11 vector were kindly gifted from Dr. Niki (NIG). S. cryophilus and S. octosporus strains were kindly gifted from N. Rhind (UMass Medical School). This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to TK (No. 15K07360), and Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry, Japan to MK. The authors thank Faculty of Life and Environmental Science in Shimane University for its help in financial supports for publishing our results.
References
- Niki H. Schizosaccharomyces japonicus: the fission yeast is a fusion of yeast and hyphae. Yeast 2014;31:83–90.10.1002/yea.v31.3
- Helston RM, Box JA, Tang W, et al. Schizosaccharomyces cryophilus sp. nov., a new species of fission yeast. FEMS Yeast Res. 2010;10:779–786.10.1111/j.1567-1364.2010.00657.x
- Conti SF, Naylor HB. Study of life-cycle of Schizosaccharomyces octosporus by means of ultra-thin sectioning and electron microscopy. Nature 1960;185:633–634.10.1038/185633a0
- Hoffman CS, Wood V, Fantes PA. An ancient yeast for young geneticists: a primer on the Schizosaccharomyces pombe model system. Genetics 2015;201:403–423.10.1534/genetics.115.181503
- Wood V, Gwilliam R, Rajandream MA, et al. The genome sequence of Schizosaccharomyces pombe. Nature 2002;415:871–880.10.1038/nature724
- Rhind N, Chen Z, Yassour M, et al. Comparative functional genomics of the fission yeasts. Science 2011;332:930–936.10.1126/science.1203357
- Klar AJ. Schizosaccharomyces japonicus yeast poised to become a favorite experimental organism for eukaryotic research. G3 (Bethesda). 2013;3:1869–1873.
- Yamada Y, Arimoto M, Kondao K. Coenzyme Q system in the classification of the ascosporogenous yeast genus Schizosaccharomyces and yeast-like genus Endomyces. J Gen Appl Microbiol. 1973;19:353–358.10.2323/jgam.19.353
- Marbois B, Xie LX, Choi S, et al. para-Aminobenzoic acid is a precursor in coenzyme Q6 biosynthesis in Saccharomyces cerevisiae. J Biol Chem. 2010;285:27827–27838.10.1074/jbc.M110.151894
- Tran UC, Clarke CF. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 2007;7(Suppl):S62–S71.10.1016/j.mito.2007.03.007
- Allan CM, Awad AM, Johnson JS, et al. Identification of Coq11, a new coenzyme Q biosynthetic protein in the CoQ-synthome in Saccharomyces cerevisiae. J Biol Chem. 2015;290:7517–7534.10.1074/jbc.M114.633131
- Kawamukai M. Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol Appl Biochem. 2009;53:217–226.10.1042/BA20090035
- Kawamukai M. Biosynthesis of coenzyme Q in eukaryotes. Biosci Biotechnol Biochem. 2016;80:23–33.
- Saiki R, Nagata A, Uchida N, et al. Fission yeast decaprenyl diphosphate synthase consists of Dps1 and the newly characterized Dlp1 protein in a novel heterotetrameric structure. Eur J Biochem. 2003;270:4113–4121.10.1046/j.1432-1033.2003.03804.x
- Saiki R, Nagata A, Kainou T, et al. Characterization of solanesyl and decaprenyl diphosphate synthases in mice and humans. FEBS J. 2005;272:5606–5622.10.1111/ejb.2005.272.issue-21
- Okada K, Suzuki K, Kamiya Y, et al. Polyprenyl diphosphate synthase essentially defines the length of the side chain of ubiquinone. Biochim Biophys Acta. 1996;1302:217–223.10.1016/0005-2760(96)00064-1
- Okada K, Kainou T, Matsuda H, Kawamukai M. Biological significance of the side chain length of ubiquinone in Saccharomyces cerevisiae. FEBS Lett. 1998;431:241–244.10.1016/S0014-5793(98)00753-4
- Suzuki K, Ueda M, Yuasa M, et al. Evidence that Escherichia coli ubiA product is a functional homolog of yeast COQ2, and the regulation of ubiA gene expression. Biosci Biotechnol Biochem. 1994;58:1814–1819.10.1271/bbb.58.1814
- Uchida N, Suzuki K, Saiki R, et al. Phenotypes of fission yeast defective in ubiquinone production due to disruption of the gene for p-hydroxybenzoate polyprenyl diphosphate transferase. J Bacteriol. 2000;182:6933–6939.10.1128/JB.182.24.6933-6939.2000
- Hayashi K, Ogiyama Y, Yokomi K, et al. Functional conservation of Coenzyme Q biosynthetic genes among yeasts, plants, and humans. PLoS ONE. 2014;9:e99038.10.1371/journal.pone.0099038
- Moriyama D, Hosono K, Fujii M, et al. Production of CoQ10 in fission yeast by expression of genes responsible for CoQ10 biosynthesis. Biosci Biotechnol Biochem. 2015;79:1026–1033.10.1080/09168451.2015.1006573
- Moriyama D, Kaino T, Yajima K, et al. Cloning and characterization of decaprenyl diphosphate synthase from three different fungi. Appl Microbiol Biotechnol. 2017;101:1559–1571.10.1007/s00253-016-7963-0
- Alfa C, Fantes P, Hyams J. Experiments with fission yeast. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993.
- Okazaki K, Okazaki N, Kume K, et al. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489.10.1093/nar/18.22.6485
- Prentice HL. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 1992;20:621.10.1093/nar/20.3.621
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823.10.1016/0076-6879(91)94059-L
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, NY.: Cold Spring Harbor Laboratory Press; 1989.
- Suzuki K, Okada K, Kamiya Y, et al. Analysis of the decaprenyl diphosphate synthase (dps) gene in fission yeast suggests a role of ubiquinone as an antioxidant. J Biochem (Tokyo). 1997;121:496–505.10.1093/oxfordjournals.jbchem.a021614
- Miki R, Saiki R, Ozoe Y, et al. Comparison of a coq7 deletion mutant with other respiration-defective mutants in fission yeast. FEBS J. 2008;275:5309–5324.10.1111/j.1742-4658.2008.06661.x
- Zhang M, Wakitani S, Hayashi K, et al. High production of sulfide in coenzyme Q deficient fission yeast. BioFactors. 2008;32:91–98.10.1002/biof.v32:1/4
- Saiki R, Ogiyama Y, Kainou T, et al. Pleiotropic phenotypes of fission yeast defective in ubiquinone-10 production. A study from the abc1Sp (coq8Sp) mutant. BioFactors. 2003;18:229–235.10.1002/biof.v18:1/4
- Kawamukai M. Biosynthesis, bioproduction and novel roles of ubiquinone. J Biosci Bioeng. 2002;94:511–517.10.1016/S1389-1723(02)80188-8
- Zhang M, Luo J, Ogiyama Y, et al. Heteromer formation of a long-chain prenyl diphosphate synthase from fission yeast Dps1 and budding yeast Coq1. FEBS J. 2008;275:3653–3668.10.1111/j.1742-4658.2008.06510.x
- Sakurai M, Tohda H, Kumagai H, Giga-Hama Y. A distinct type of alcohol dehydrogenase, adh4+, complements ethanol fermentation in an adh1-deficient strain of Schizosaccharomyces pombe. FEMS Yeast Res. 2004;4:649–654.10.1016/j.femsyr.2003.12.009
- Bulder CJ. Anaerobic growth, ergosterol content and sensitivity to a polyene antibiotic, of the yeast Schizosaccharomyces japonicus. Antonie van Leeuwenhoek. 1971;37:353–358.10.1007/BF02218505
- Naehring J, Kiefer S, Wolf K. Nucleotide sequence of the Schizosaccharomyces japonicus var. versatilis ribosomal RNA gene cluster and its phylogenetic implications. Curr Genet. 1995;28:353–359.10.1007/BF00326433
- Svoboda A, Slaninova I. Colocalization of microtubules and mitochondria in the yeast Schizosaccharomyces japonicus var. versatilis. Can J Microbiol. 1997;43:945–953.10.1139/m97-136
- Okada K, Ohara K, Yazaki K, et al. The AtPPT1 gene encoding 4-hydroxybenzoate polyprenyl diphosphate transferase in ubiquinone biosynthesis is required for embryo development in Arabidopsis thaliana. Plant Mol Biol. 2004;55:567–577.
- Aoki K, Nakajima R, Furuya K, et al. Novel episomal vectors and a highly efficient transformation procedure for the fission yeast Schizosaccharomyces japonicus. Yeast 2010;27:1049–1060.10.1002/yea.v27.12