Abstract
White sword bean (Canavalia gladiata) seeds have the potential to be utilized in the manufacturing of processed foods owing to their high protein and carbohydrate content. Our previous reports explored the use of the sword bean as a source of food materials by preparing extracts in distilled water. In the present study, we found that one such extract can be gelated by cooling. The gelling substances were extracted by boiling and simultaneously stirring a suspension containing ground beans. Few proteins were present in the gelated extract. We also examined the conditions under which gelation occurred and the gel melting temperature. The extract gelated at temperatures below 10 °C, and the resulting gel melted at those above 65 °C. This is the first report that gelling substances can be extracted from sword beans in large quantities. We expect that this gelling agent can be used for the production of processed foods.
A crude sword bean (Canavalia gladiata) extract is gelated by cooling.
The sword bean (Canavalia gladiata) is a leguminous plant that originated in the Asian continent and subsequently spread throughout the tropics. Eaten as a green vegetable in Asia [Citation1], this plant has particular agronomic traits, including a high cultivation temperature (15–30 °C). Moreover, its average yield is comparable to that of the soybean [Citation2], and it is relatively resistant to pests and diseases [Citation3]. Concerning nutritional properties, sword beans contain approximately 26% protein, 3% fat, and 62% carbohydrate [Citation4]. These characteristics make the sword bean of potential use in the preparation of processed foods.
In our previous study, we prepared three sword bean extracts (Extract A, B, and C) using our own methods established for this purpose (Supplemental Figure 1) [Citation5]. For the preparation of Extract A, soaked beans were ground in distilled water and separated into the extract and waste following heating. Extract B was prepared in the same manner but without heating. Finally, Extract C was produced by heating Extract B and removing the resulting precipitates. We found that Extract B contains a large amount of proteins [Citation5] and its preparation represents an inexpensive and simple method for the purification of the major protein present, canavalin [Citation6], which has a high leucine content [Citation7,8]. The characteristics of Extract B make it a suitable source for the extraction of proteins, and the other extracts obtained may also be useful for the production of processed foods.
In the present study, we observed that Extract A gelates when cooled. Moreover, we established a novel method for the extraction of gelling substances from dried sword beans by carefully examining experimental conditions. In addition, the temperature at which these substances gelate and that at which the resultant gel melts were also determined. This work provides information that will contribute to the utilization of the sword bean in the food industry.
Materials and methods
Materials
White sword beans were purchased from Morika Beiten (Nara, Japan), and general chemical reagents and starch and cellulose were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Preparation of sword bean extracts
Sword bean extracts (Extract A and C) were prepared according to previously described methods (Supplemental Figure 1) [Citation5]. Dried sword beans were soaked in 10 volumes (v/w) of distilled water at 20 °C for 18 h. The soaked beans were then ground on ice for 5 min in 8 volumes (v/w) of distilled water using a hand blender (CSB-77JBSTRW, Cuisinart, Stamford, CT, USA). The suspension containing ground beans was incubated at 100 or 105 °C without stirring in a block bath (CDB-105, AS ONE, Osaka, Japan) for 3 min, or was boiled with gentle stirring on a heated stir plate (RET control-visc, IKA, Staufen, Germany) for 3 min. The ground beans were removed from the heated suspension by sieving through a cotton cloth. The filtrate, which corresponds to Extract A described in our previous reports, was separated into supernatant and precipitate by centrifugation at 9100 × g at 20 °C for 10 min. In another protocol, the ground beans were first removed from the suspension by sieving through a cotton cloth prior to the application of heat. The filtrate was boiled as described above, and the mixture was again sieved through a cotton cloth. This filtrate corresponds to Extract C in our previous reports and was subsequently separated into supernatant and precipitate by centrifugation at 9100 × g at 20 °C for 10 min.
Analysis of gelation
Extracts were prepared as above for the determination of gelation conditions. Samples were incubated at 20 or 4 °C for 1 day and separated into supernatant and precipitate by centrifugation at 9100 × g at 20 °C for 10 min. Wet precipitate weights were measured with an electronic balance (HR-120, A&D Company, Tokyo, Japan), and gelation efficiency was calculated as the percentage of the initial sample weight represented by the wet precipitate weight. Data are presented as the average of three independent experiments.
Analysis of dry weight of gelling substance
Samples were incubated at 20 or 4 °C for 1 day and separated into supernatant and precipitate by centrifugation at 9100 × g at 20 °C for 10 min. The supernatant was removed. The wet precipitate was dried at 105 °C for 3 h until a constant weight was reached. The dry precipitate weight was measured with the same balance and was converted into that per 1 g of dried bean. Data are presented as the average of five independent experiments.
Analysis of optimal incubation time for gelation
The suspension containing ground beans was boiled with stirring and used to determine the optimal incubation time for gelation. Samples were incubated at 20 or 4 °C for various lengths of time and evaluated as described above.
Analysis of gelation temperature
The suspension containing ground beans was boiled with stirring and used for analysis of gelation temperature. Samples were incubated at various temperatures for 2 days and separated into supernatant and precipitate by centrifugation at 9100 × g at 20 °C for 10 min. Wet precipitate weights were measured and gelation efficiency was estimated as above. Data are presented as the average of three independent experiments.
Analysis of gel melting temperature
The precipitates prepared by incubation at 4 °C for 2 days were incubated at various temperatures for 5 min, before being centrifuged at 9100 × g at 20 °C for 10 min. Wet precipitate weights and gelation efficiency were then calculated as above. Data are shown as the average of three independent experiments.
SDS-PAGE
Samples were mixed with 0.33 volumes of SDS sample buffer (0.25 M Tris-HCl [pH 7.0], 4% SDS, 5% 2-mercaptoethanol, and 40% glycerol) and incubated at 100 °C for 5 min. SDS-PAGE was carried out on 10% polyacrylamide gels at a constant current of 12.5 mA for 2.5 h according to the standard method described by Laemmli [Citation9]. Proteins were stained with 0.25% Coomassie Brilliant Blue R-250. The molecular weight standard was purchased from Life Technologies (Tokyo, Japan).
Iodo-starch reaction
The iodo-starch reaction was carried out according to the method described by Reid et al., with minor modifications [Citation10]. Various sword bean extracts were prepared using different procedures. Soaked beans were ground in distilled water as described above, before being separated into supernatant and precipitate by centrifugation. The supernatant was then tested directly or following boiling. In a separate protocol, suspensions containing ground beans were boiled and then sieved through a cotton cloth, as described above. The filtrate was subsequently separated into supernatant and precipitate, also as above. A suspension of 1% starch in distilled water served as a positive control. To each supernatant, distilled water as a negative control, and the positive control, 0.025 volumes of iodine-potassium iodide solution was added and the reactions were mixed well.
Statistical analyses
The t-test was used to compare means among groups in the experiment testing the effect of incubation temperature and in the experiment for dry weight. For the extract procedure and extract incubation temperature experiments, a one-way analysis of variance and Bartlett test were employed to compare group means. Post hoc analysis was performed with the Tukey–Kramer test when analysis of variance indicated a significant difference. Differences associated with p values < 0.05 were considered statistically significant.
Results
Gelation of sword bean extract
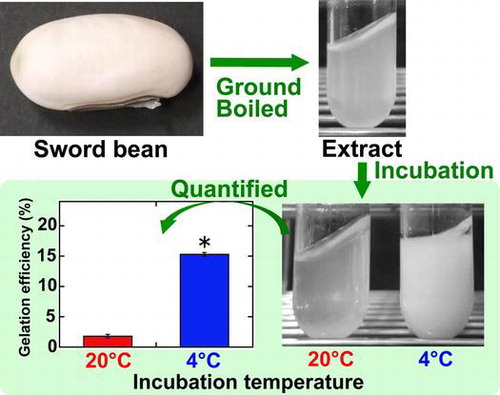
Sword bean extract was prepared by boiling with ground beans present, before sieving through a cotton cloth. The extract was then incubated at 20 or 4 °C for 1 day (Figure ). The extract initially appeared slightly cloudy (Figure (A), a); however, after incubation at 4 °C, it became opaque and solidified (Figure (A), c). Incubation at 20 °C did not have this effect (Figure (A), b). Following centrifugation of these incubated samples, gelation efficiency was calculated to be 15.3 ± 0.3% for incubation at 4 °C, and 1.8 ± 0.3% for that at 20 °C (Figure (B)), and this difference was found to be significant (p < 0.001). These results show that sword bean extract can be gelated by incubation at 4 °C, representing the first report of the gelation of an extract from this plant.
Figure 1. Gelation of sword bean extract. (A) Soaked sword beans were ground in distilled water. The suspension containing ground beans was then boiled and sieved through a cotton cloth (a). The extract was incubated at 20 °C (b) or 4 °C (c) for 1 day. The photographs of the extracts were taken while inclining the test tubes at 45°. (B) After incubation, the gelation efficiency (the weight of the wet precipitate as a percentage of that of the initial sample) was calculated. Data are expressed as the means ± standard deviations of three independent experiments. The statistical significance of differences was determined by the t-test. *p < 0.001.
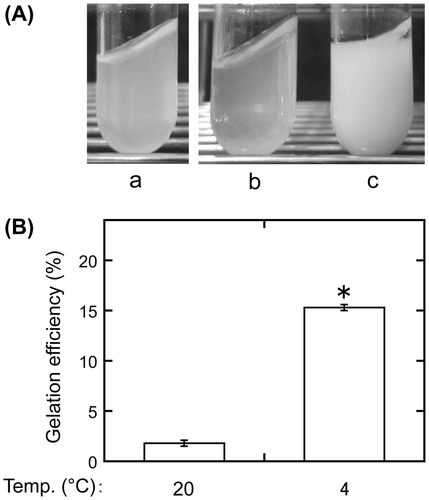
Figure 2. Effects of heating treatment during extract preparation on gelation. Sword bean extracts were prepared by incubating the suspension containing ground beans at 100 or 105 °C for 3 min or boiling it with stirring for 3 min. The heated suspension was sieved through a cotton cloth, and the extracts were incubated at 20 or 4 °C for 1 day. Gelation efficiency was calculated as in Figure . Data are expressed as the means ± standard deviations of three independent experiments. The statistical significance of differences was determined by one-way analysis of variance and the Tukey–Kramer test. Different letters indicate a significant difference (p < 0.001).
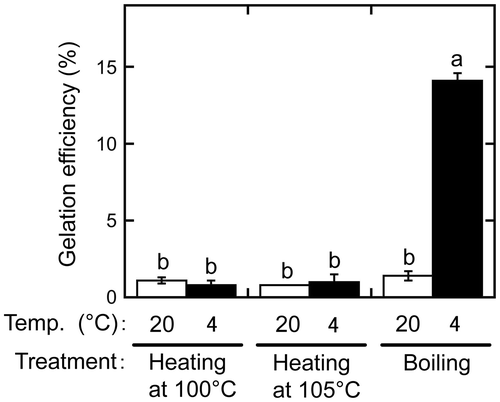
Figure 3. Difference between extracts produced by removing ground beans before and after boiling. One extract was produced by boiling a suspension containing ground beans, which were then removed by sieving (+). Another was prepared by removing the ground beans from the suspension before boiling, after which, it was sieved again (−). The extracts were then incubated at 20 °C (white bars) or 4 °C (black bars) for 1 day. Gelation efficiency was calculated as in Figure . Data are expressed as the means ± standard deviations of three independent experiments. Statistically significant differences were determined by one-way analysis of variance and the Tukey–Kramer test. Different letters indicate a significant difference (p < 0.001).
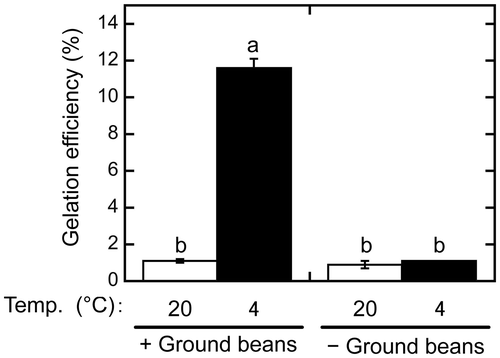
Figure 4. Analysis of sword bean proteins in extracts prepared using different procedures. The effects of each extraction protocol on sword bean proteins were analyzed by SDS-PAGE. During extraction, suspensions containing ground beans (+) (lanes 1, 3, and 4) or not (−) (lanes 2, 5, and 6) were boiled (+) (lanes 3–6) or not (−) (lanes 1 and 2). The boiled suspensions were then sieved to obtain extracts, whereas the unboiled suspensions were not, instead being used directly as extracts. In addition, the extracts were centrifuged (+) (lanes 4 and 6) or not (−) (lanes 1–3, and 5).
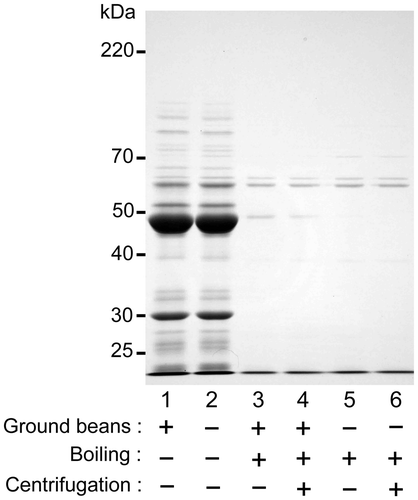
Figure 5. Effect of incubation time on gelation. An extract was prepared by boiling and subsequently sieving a suspension containing ground beans. The extract was then incubated at 4 °C (open circles) or 20 °C (closed circles) for between 0 and 14 days. Gelation efficiency was calculated as in Figure . Data are expressed as the means ± standard deviations of three independent experiments.
In addition, the incubated samples were dried following the removal of supernatant. The dry weight was calculated to be approximately 71.5 ± 0.4 mg/g dried bean for incubation at 4 °C and 8.1 ± 0.2 mg/g dried bean for that at 20 °C (p = 0.730 × 10−17). The results indicate that gelling substance is extracted in the dry weight of approximately 63 mg/g dried bean. However, since the gelling substance is crude, the dry weight is a rough indication.
Effects of extraction procedure on gelation
To investigate the effects of heating conditions on gelation, extracts were prepared using various heating methods, before being sieved through a cotton cloth (Figure ). Only when the extract was incubated at 4 °C after boiling with stirring did gelation efficiency dramatically increase (to 14.1 ± 0.5%, p < 0.001). When the extract was incubated at 20 °C, heating conditions had little effect on gelation efficiency, which was 1.1 ± 0.2% for heating at 100 °C, 0.8 ± 0.0% for heating at 105 °C, and 1.4 ± 0.3% for boiling with stirring. In addition, when the extract was prepared by heating at 100 or 105 °C before being incubated at 4 °C, gelation efficiency was negligible, being 0.8 ± 0.3% for the former and 1.0 ± 0.5% for the latter. These results indicate that boiling is an essential factor in the preparation of gelling substances from sword beans.
In a previous study, we demonstrated that the extract produced by removing the ground beans before applying heat contains a large amount of proteins, most of which are precipitated by heating at temperatures above 90 °C [Citation5]. In the procedures described above, the extract was prepared by boiling without first removing the ground beans. To investigate whether an extract prepared as in our previous study can be gelated after boiling, the ground beans were removed by sieving through a cotton cloth before boiling, after which, the extract was incubated at 4 °C and centrifuged (Figure ). Interestingly, this extract did not form a gel when incubated at 4 °C, with a gelation efficiency of 1.1 ± 0.0%. In contrast, the gelation efficiency of the extract prepared by boiling with ground beans present was significantly increased by incubation at 4 °C (11.6 ± 0.5%, p < 0.001). These results indicate that retaining ground beans during boiling is essential for the preparation of gelling substances from sword beans.
In our previous work, we identified that certain seed-derived proteins form a gel when cooled to 4 °C following heating [Citation11]. Thus, in the current investigation, we analyzed the proteins in each sword bean extract by SDS-PAGE (Figure ). Prior to boiling, the extracts contained large amounts of protein, whether the ground beans were present (lane 1) or not (lane 2). In contrast, extracts contained few proteins after boiling (lanes 3–6), also regardless of the inclusion of ground beans in the boiling mixture (lanes 3 and 4). As shown in Figure , gelation of the extract was dependent on inclusion of the ground beans during boiling. These results indicate that proteins are not the main components of the gelling agents in sword beans.
Effect of incubation conditions on gelation
To determine the optimal incubation time for gelation, the extract prepared by boiling with ground beans was incubated at 4 or 20 °C for 2 weeks (Figure ). The gelation efficiency of the extract incubated at 4 °C markedly increased (to 27.2 ± 1.1%) after 1 day, and reached a maximum of 32.5 ± 0.5% after 2 days, before plateauing (Figure , open circles). In contrast, the gelation efficiency of the extract incubated at 20 °C only slightly increased (to 5.8 ± 1.6%) after 14 days (Figure , closed circles). These observations indicate that maximum gelation of sword bean extract is achieved after 2 days.
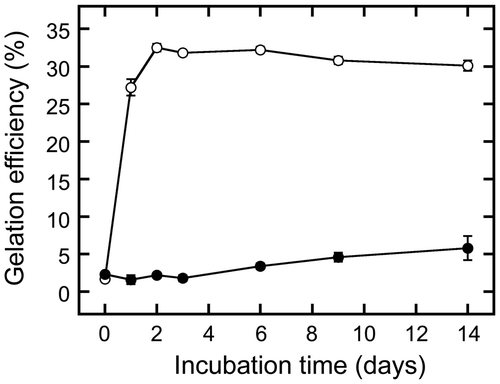
As described above, the extract was gelated by cooling to 4 °C. As gelation temperature is an important factor in food processing, we determined the maximum temperature at which gelation could be induced (Figure ). Each extract was incubated at a temperature between 4 and 20 °C for 2 days. Gelation efficiency was considerably higher at 10 °C (14.6 ± 0.2%) than at higher temperatures, and was gradually increased by decreasing the temperature (being 21.9 ± 0.1% at 4 °C). These changes in gelation efficiency demonstrate that sword bean extract gelates when incubated at less than 10 °C, and that this process is promoted by further decreasing the incubation temperature.
Figure 6. Effect of temperature on gelation. Extracts were prepared by boiling and then sieving suspensions containing ground beans. Each was then incubated at a temperature between 4 and 20 °C for 2 days. Gelation efficiency was calculated as in Figure . Data are expressed as the means ± standard deviations of three independent experiments.
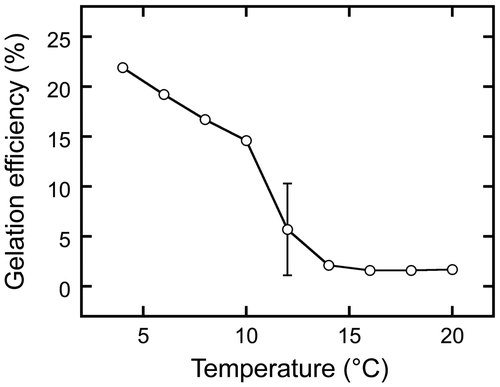
Figure 7. Establishment of gel melting temperature. Gels were prepared from sword bean extracts by incubation at 4 °C for 2 days. Each was then incubated at a temperature between 20 and 100 °C for 5 min. Gelation efficiency was calculated as in Figure . Data are expressed as the means ± standard deviations of three independent experiments.
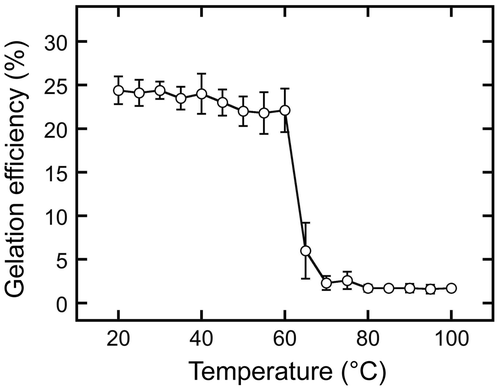
Figure 8. Iodo-starch reactions to sword bean extracts prepared using different procedures. Soaked sword beans were ground and separated into supernatant (b) and precipitate by centrifugation. The supernatant was then boiled (c). Separately, an extract was prepared by boiling suspensions containing ground sword beans and sieving them through a cotton cloth (d). Each extract, distilled water (a), and 1% starch (e) were then mixed with iodine-potassium iodide solution.

Gel melting temperature
Of the various physicochemical properties of gels, melting temperature is also of importance in the production of processed foods. We therefore investigated the melting temperature of the gel (Figure ) prepared from sword bean extract by incubation at 4 °C for 2 days. The gel was incubated at a temperature between 20 and 100 °C for 5 min. Gelation efficiency was substantially lower at 65 °C (6.0 ± 3.2%) than at 4 °C (21.9 ± 0.1%), and plateaued at approximately 2% at 70 °C and above. These findings indicate that the gel obtained melted when exposed to temperatures greater than 65 °C.
Iodo-starch test of sword bean extracts
Various sword bean extracts were analyzed with the iodo-starch reaction (Figure ). No reaction was observed using the supernatant of the sword bean extract from which the ground beans had been removed (Figure , b), even after it had been boiled (Figure , c); however, slight coloration was noted using the extract prepared by boiling with the ground beans present (Figure , d), i.e. that which gelated when cooled to 4 °C. In contrast, strong color development was apparent when an unboiled 1% starch suspension was tested (Figure , e). Given the data depicted in Figure (B), which show a gelation efficiency of 15.3 ± 0.3%, it seems likely that the substances responsible for gelation represent more than 1% of the extract. Thus, these substances are unlikely to be starches.
Discussion
Gel-forming molecules are utilized in various processed foods as gelation agents, stabilizers, and thickeners. For instance, gelatin, agar, pectin, and carrageenan are used as thickening stabilizers in jelly, pudding, dressing, sauce, and yogurt, among other products. In this study, we found that sword bean extract gelated at 4 °C, representing the first report that this bean is a source of plant-derived gelling agents.
Gelling substances are broadly separated into proteins and polysaccharides. Examples of the former include proteins from egg white [Citation12, soybean [Citation13,14], sesame, and perilla [Citation11,15], and those of the latter include agar [Citation16], pectin [Citation17], and carrageenan [Citation16]. This begs the question of whether the major gelling substance in sword bean extract is a protein or a polysaccharide. Our SDS-PAGE results indicated that few proteins were present in the boiled sword bean extract (Figure , lanes 3 and 4). In addition, our previous study demonstrated that most sword bean proteins are removed from the extract by centrifugation following heating at more than 90 °C [Citation5]. However, gelation efficiency was approximately 15% when the extract was boiled with stirring in the present work (Figure). It is unlikely that a small amount of protein would result in such high gelation efficiency, suggesting that the major gelling agent is non-proteinous. Our preliminary experiments suggest that the gelation substances are polysaccharides (unpublished data); however, extracts of higher purity are required to determine their chemical composition.
Sword beans contain abundant carbohydrates [Citation4,18], and the starch yield from the dehulled seed is 31% [Citation18]. To explore the possibility that the major gelling substance is starch, sword bean extracts were analyzed using the iodo-starch reaction (Figure ). Only the extract that gelated after cooling to 4 °C (with a gelation efficiency of approximately 15%) exhibited slight coloration (Figure , d). However, the strength of this reaction was far lower than that observed with 1% starch (Figure , e). It does not seem feasible that a gelation efficiency of 15% could result from the presence of starch at a concentration of less than 1%. Moreover, an iodo-starch test of boiled starch solution (Supplemental Figure 2, c) resulted in a color similar to that observed with untreated starch (Supplemental Figure 2, b and c). These results imply that the major gelling substance is not starch and is not detectable using the iodo-starch reaction. In addition, these results show that little sword bean starch was extracted using our methods. We also examined the possibility that cellulose, a typical plant-derived polysaccharide, is the main sword bean component responsible for gelation (Supplemental Figure 2, d and e). Neither the untreated nor the heated cellulose sample reacted visibly in the iodo-starch test. Furthermore, cellulose is almost insoluble in water, even when boiled [Citation19]. As shown in Supplemental Figure 2, most of the cellulose was in a solid state in these samples, whether boiling was carried out (d) or not (e). In contrast, the sword bean gelling substances dissolved in distilled water when boiled (Figure ). This discrepancy in solubility precludes the possibility that the principal gelling agent is cellulose. Considering our results together, we deduce that the gelling substances are not starches or celluloses, but other polysaccharides.
The gelling substance gelated at temperatures below 10 °C (Figure ) and the resulting gel melted at those above 65 °C (Figure ). This difference between gelation and melting temperatures demonstrates that the major gelling substance displays thermal hysteresis. In approximate terms, agar gelates at less than 35 °C and melts at more than 80 °C [Citation20]. Thus, the gelation temperature of the gelling substance from sword beans is lower than that of agar, and the difference between its gelation and melting temperatures is larger. In addition, it does not gelate at room temperature (Figure ). Therefore, this gelling agent is suitable for use in food processing. However, the nature of this substance remains unclear owing to the crudity of the extract tested.
Extracts of higher purity are required to identify the substance and determine its characteristics. The first step of the extraction comprised grinding sword beans in distilled water, followed by separation of the extract from the ground beans. Our results suggest that the gelling substance derives from the ground beans themselves (Figure ). Separation will be a crucial step for the establishment of an improved procedure for the extraction of the gelling substance, and is a feasible process from the perspective of food industry applications. We previously reported the establishment of a method for the extraction of sword bean proteins [Citation5], which are found in abundance in the extract that remains after sieving out the ground beans with a cotton cloth. The residual ground beans might thus be considered waste; however, our present report raises the possibility that they may also be of use, particularly for the extraction of gelling substances.
Stirring during boiling appears to be an important factor for the extraction of gelling substances from sword beans, as the different heating conditions tested here brought about different results (Figure ). Cooling resulted in gelation when the sword bean suspension was boiled with stirring, but not when it was incubated at 100 or 105 °C without stirring.
In conclusion, we found that a crude sword bean extract could be gelated, representing the first report of a gelling substance being present in this plant. A method of crude extraction was established and the conditions under which gelation of the crude extract occurs were determined in detail. Gelation of the extracted gelling substance occurred at 10 °C and the resulting gel melted at temperatures above 65 °C. In addition, the presence of ground beans is an essential factor for extraction of the gelling substance by boiling. Although the nature of this substance remains unclear, it is evident that it can be extracted from sword beans in large quantities. In future work, we will prepare high-purity extracts in order to identify this substance, which we expect will be of use in food processing, pharmaceuticals, drug delivery, and tissue engineering, among other applications.
Author contributions
YA conceived and designed the experiments. YA and KN performed the experiments. KN analyzed the data and wrote the paper. YA reviewed and edited the manuscript. KN and YA read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by authors.
Supplemental data
Supplemental data for this article can be accessed https://doi.org/10.1080/09168451.2017.1403884
Supplemental_fig2.pdf
Download PDF (69.4 KB)Supplemental_fig1.pdf
Download PDF (65.9 KB)Acknowledgements
We thank Misaki Takahashi and Ayaka Nishiura for their technical assistance. We would like to thank Editage (www.editage.jp) for English language editing.
References
- Purseglove JW. Tropical crops: dicotyledons. London: Longman; 1974.
- Bressani R, Brenes RG, García A, et al. Chemical composition, amino acid content and protein quality of Canavalia spp. seeds. J Sci Food Agric. 1987;40:17–23.10.1002/(ISSN)1097-0010
- Smartt J. Tropical pulses. London: Longman; 1976.
- Vadivel V, Janardhanan K. Nutrition and antinutritional characteristics of seven south Indian wild legumes. Plant Foods Hum. Nutr. 2005;60:69–75.10.1007/s11130-005-5102-y
- Nishizawa K, Masuda T, Masui H, et al. Precipitation of sword bean proteins by heating and addition of magnesium chloride in a crude extract. Biosci Biotechnol Biochem. 2016;80:1623–1631.10.1080/09168451.2016.1164587
- Nishizawa K, Arii Y. Reversible changes of canavalin solubility controlled by divalent cation concentration in crude sword bean extract. Biosci Biotechnol Biochem. 2016;80:2459–2466.10.1080/09168451.2016.1224642
- Yamauchi D, Nakamura K, Asahi T, et al. cDNAs for canavalin and concanavalin A from Canavalia gladiata seeds. Nucleotide sequence of cDNA for canavalin and RNA blot analysis canavalin and concanavalin A mRNAs in developing seeds. Eur J Biochem. 1988;170:515–520.10.1111/ejb.1988.170.issue-3
- Takei Y, Yamauchi D, Minamikawa T. Nucleotide sequence of the canavalin gene from Canavalia gladiata seeds. Nucleic Acids Res. 1989;17:4381.10.1093/nar/17.11.4381
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.10.1038/227680a0
- Reid MS, Padfield CAS, Watkins CB, et al. Starch iodine pattern as a maturity index for Granny Smith apples. N Z J Agric Res. 1982;25:229–237.10.1080/00288233.1982.10420918
- Takenaka Y, Arii Y, Masui H. Subuit structure and functional properties of the predominant globulin of perilla (Perilla frutescens var. frutescens) seeds. Biosci Biotechnol Biochem. 2010;74:2475–2479.10.1271/bbb.100557
- Funaki J, Minami M, Abe S, et al. Effect of proteolytic modification on texture and mastication of heat-treated egg white gels. J Food Process Preserv. 2017;41:e12857.10.1111/jfpp.2017.41.issue-1
- Arii Y, Takenaka Y. Magnesium chloride concentration-dependent formation of tofu-like precipitates with different physicochemical properties. Biosci Biotechnol Biochem. 2013;77:928–933.10.1271/bbb.120864
- Arii Y, Takenaka Y. Initiation of protein association in tofu formation by metal ions. Biosci Biotechnol Biochem. 2014;78:86–91.10.1080/09168451.2014.877341
- Takenaka Y, Arii Y, Masui H. Network structure and forces involved in perilla globulin gelation: comparsion with sesame globulin. Biosci Biotechnol Biochem. 2011;75:1198–1200.10.1271/bbb.110046
- Tako M. The principle of polysaccharide gels. Adv Biosci Biotechnol. 2015;6:22–36.10.4236/abb.2015.61004
- Abid M, Cheikhrouhou S, Renard CM, et al. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2017;215:318–325.10.1016/j.foodchem.2016.07.181
- Adebowale KO, Afolabi TA, Olu-Owolabi BI. Functional, physicochemical and retrogradation properties of sword bean (Canavalia gladiata) acetylated and oxidized starches. Carbohydr Polym. 2006;65:93–101.10.1016/j.carbpol.2005.12.032
- Olson A, Gray GM, Chin M. Chemistry and analysis of soluble dietary fiber. Food Technol. 1987;41:71–80.
- Shintani S, Hori Y, Yamanouchi T, et al. Kaseigaku zasshi [Physical properties of gelatin-agar jelly]. 1975;26:271–276. Japanese.
