Abstract
A novel acetal named isogloiosiphone B was isolated from the red alga Neodilsea yendoana, along with three known hydrophobic compounds as β-glucuronidase inhibitors. The acetal was determined as a naturally occurring compound from the extraction experiments with several kinds of solvent. The acetal showed the highest inhibition against β-glucuronidase among the compounds examined.
Beta-glucuronidase (EC 3.2.1.31) is not only found in anaerobic intestinal bacteria [Citation1], but also in mammalian organs and body fluids [Citation2–5]. The bacterial enzyme catalyzes hydrolysis of β-glucuronosyl-O-bond [Citation6] to generate β-glucuronic acid and aglycons. The aglycons can be re-absorbed into body to delay eliminating toxic compounds from body as glucuronides [Citation7]. Excess expression of β-glucuronidase in human is related with diverse pathological symptoms [Citation8–13]. Hence, β-glucuronidase inhibitors are highly expected to sustain healthy condition. Neodilsea yendoana Tokida (Dumontiaceae, Gigartinales) is a red macroalga abundantly distributed along the coast of North Japan. Several studies have revealed that N. yendoana comprises amino acids [Citation14], rhodoic acid [Citation15], polyunsaturated fatty acid [Citation16], and yendolipin [Citation17]. The present paper aims to isolate novel potential β-glucuronidase inhibitors from N. yendoana.
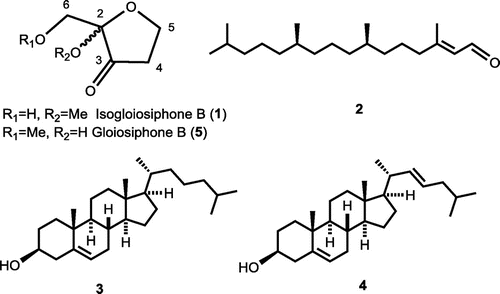
Methanol extract of air-dried N. yendoana (1.73 kg), collected along the coast of Hakodate, Japan in 2016, was separated to ethyl acetate-soluble fraction (1.30 g) by organic solvent partitioning with guidance of results by slightly modified Escherichia coli β-glucuronidase inhibition assay using p-nitrophenyl β-D-glucuronide (Supplemental method 1) [Citation18]. The fraction was chromatographed twice on silica gel and Sephadex LH-20 to obtain three inhibitory fractions. Final purification was performed using HPLC (ULTRON VX-ODS, 5 μm; 250 × 4.6 mm) eluted with MeCN-H2O = 15:85 and MeCN-H2O = 9:1 to obtain novel compound 1 (1.43 mg), and phytal (2, 2.02 mg), which was first isolated from this alga, cholesterol (3, 3.08 mg), and 22-dehydrocholesterol (4, 3.31 mg), respectively (Supplemental method 2). Compounds 2–4 were identified by comparison of literature data [Citation19,20].
Novel compound 1 was obtained as colorless oil. Physicochemical data for 1 were as follows: UV λmax (log ε) (MeOH) 206 (2.60); IR νmax (CHCl3): 1769, 1081 cm−1; [α]D27 = 0 (c1.32, CHCl3); FI-MS m/z 146.07 (M+); FI-HR-MS m/z 146.05861 (M+, calcd 146.05791 for C6H10O4); 1H NMR (500 MHz, acetone-d6) and 13C NMR (125 MHz, acetone-d6): see Table . IR spectrum of 1 showed C=O and C-O stretching peaks. 1H NMR spectrum of 1 indicated the presence of one hydroxy, three methylene, and one methoxy proton signals, where the hydroxy proton was deduced from no cross-peak in HSQC spectrum. 13C NMR spectrum of 1 revealed one carbonyl, one quaternary, three methylene, and one methoxy carbon signals which were assisted with HSQC experiment. DQF COSY experiment of 1 revealed two couples of correlations (H-4/H-5 and H-6/hydroxy proton). The latter correlation was assigned as a hydroxymethyl group. All the proton and carbon signals of compound 1 were assigned according to HMBC cross-peaks of C-2 with Hs-5 and 6, and methoxy protons; ketonic carbon (C-3) with Hs-4, 5 and 6; C-4 with Hs-5; C-5 with H-4; and C-6 with the hydroxy proton. Consequently, compound 1 was elucidated as an isomer of known compound gloiosiphone B (5) [Citation21], a structural analog of laurencione [Citation22], isolated from the red alga Gloiosiphonia verticillaris (Gloiosiophoniaceae, Gigartinales). The 13C NMR signal at C-2 of 1 was shifted to downfield compared to the signal of gloiosiphone B while the signal at C-6 of 1 was shifted to up-field. This difference was contributed to substituting position of methoxy group. Thus, compound 1 was identified as 2-hydroxymethyl-2-methoxyoxolan-3-one, a novel acetal named isogloiosiphone B (Figure ). Compound 1 possesses an asymmetric center in its structure. However, 1 might be a racemic mixture because specific rotation of 1 was optically inactive. With the consideration that compound 1 might be an artifact from its simple acetal structure, the alga was extracted with methanol, ethanol, and acetone. Compound 1 was isolated from all the extracts using different solvents (Figures S7–S9). Thus compound 1 was determined as a naturally occurring compound, not an artifact. Plausible precursor of isogloiosiphone B and gloiosiphone B would be 1,5-dihydroxypentane-2,3-dione. After cyclization of the precursor to corresponding hemiacetal, both compounds may be derived by methylation at different positions.
Table 1. 1H (500 MHz) and 13C NMR (125 MHz) data of compound 1 and literature data [Citation21] of gloiosiphone B (5).
Figure 1. Compounds 1–4 isolated from N. yendoana as β-glucuronidase inhibitors and gloiosiphone B (5).
The isolated compounds 1–4 were evaluated for inhibitory effects against E. coli β-glucuronidase (Table , Supplemental method 3). Isogloiosiphone B (1) showed the highest inhibitory activity in a competitive manner (Figure S19) among the isolated compounds and the positive control D-glucaro-1,4-lactone [Citation23]. Compound 1 might easily access to active site of the enzyme. Additionally, this is the first report that phytal (2), cholesterol (3) and 22-dehydrocholesterol (4) exhibited β-glucuronidase inhibitory activity even though their inhibitory activities were weak.
Table 2. β-Glucuronidase inhibitory activities of compounds 1–4.
In summary, we isolated compounds 1–4 as β-glucuronidase inhibitors from the red alga N. yendoana. Compound 1 was determined as a novel naturally acetal and showed strong β-glucuronidase inhibitory activity. In addition, this is the first report on isolation of phytal from the alga and elucidation of β-glucuronidase inhibitory activity by the hydrophobic compounds.
Author contribution
D. Z. performed all experiments and prepared draft. H. K. made experimental plans and organized all of the studies. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by authors.
Supplemental data
The supplemental material for this paper is available at https://doi.org/10.1080/09168451.2017.1403885.
supplement_datarev1.pptx
Download MS Power Point (1,001.8 KB)Acknowledgment
We thank to Dr. Eri Fukushi and Mr. Yusuke Takata, GC-MS & NMR Laboratory, Faculty of Agriculture, Hokkaido University, for measurements of MS and NMR spectra.
References
- Khan KM, Saad SM, Shaikh NN, et al. Synthesis and beta-glucuronidase inhibitory activity of 2-arylquinazolin-4(3H)-ones. Bioorg Med Chem. 2014;22:3449–3454.10.1016/j.bmc.2014.04.039
- Oleson L, Court MH. Effect of the β-glucuronidase inhibitor saccharolactone on glucuronidation by human tissue microsomes and recombinant UDP-glucuronosyltransferases (UGTs). J Pharm Pharmacol. 2008;60(9):1175–1182.10.1211/jpp.60.9.0009
- Boyer MJ, Tannock IF. Lysosomes, lysosomal enzymes, and cancer. Adv Cancer Res. 1992;60:269–291.10.1016/S0065-230X(08)60828-3
- Marsh CA, Alexander F, Levvy GA. Glucuronide decomposition in the digestive tract. Nature. 1952;170:163–164.10.1038/170163a0
- Swank RT, Pfister K, Miller D, et al. The egasyn gene affects the processing of oligosaccharides of lysosomal beta-glucuronidase in liver. Biochem J. 1986;240:445–454.10.1042/bj2400445
- Sperker B, Backman JT, Kroemer HK. The role of beta-glucuronidase in drug disposition and drug targeting in humans. Clin Pharmacokinet. 1997;33(1):18–31.10.2165/00003088-199733010-00003
- Taha M, Ismail NH, Imran S, et al. Synthesis of benzimidazole derivatives as potent β-glucuronidase inhibitors. Bioorg Chem. 2015;61:36–44.10.1016/j.bioorg.2015.05.010
- Ronald AR, Silverblatt F, Clark H, et al. Failure of urinary beta-glucuronidase activity to localize the site of urinary tract infection. Appl Microbiol. 1971;21(6):990–992.
- De Graaf M, Boven E, Scheeren H, et al. Beta-glucuronidase-mediated drug release. Cur Pharm Des. 2002;8(15):1391–1403.10.2174/1381612023394485
- Bramwell K, Ma Y, Weis JH, et al. Lysosomal β-glucuronidase regulates lyme and rheumatoid arthritis severity. J Clin Invest. 2014;124(1):311–320.10.1172/JCI72339
- Ohta H, Ono M, Sekiya C, et al. Serum immunoreactive beta-glucuronidase determined by an enzyme-linked immunosorbent assay in patients with hepatic diseases. Clin Chim Acta. 1992;208(1–2):9–21.10.1016/0009-8981(92)90019-M
- Salar U, Khan KM, Taha M, et al. Biology-oriented drug synthesis (BIODS): in vitro β-glucuronidase inhibitory and in silico studies on 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl aryl carboxylate derivatives. Eur J Med Chem. 2017;125:1289–1299.10.1016/j.ejmech.2016.11.031
- Jacox RF, Feldmahn A. Variations of beta glucuronidase concentration in abnormal human synovial fluid. J Clin Invest. 1955;34(2):263–267.10.1172/JCI103079
- Kuriyama M. Ninhydrin reactive substances in marine algae-I. On the absorbable fraction on strong cationic ion-exchange resin. Bull Jpn Soc Sci Fish. 1961;27(7):689–693.10.2331/suisan.27.689
- Murakoshi I, Hatanaka SI. Sulfur-containing amino acids in nature. Yuuki Gosei Kagaku. 1977;35(5):343–353. Japanese.
- Suzuki M, Wakana I, Denboh T, et al. An allelopathic polyunsaturated fatty acid from red algae. Phytochemistry. 1996;43(1):63–65.10.1016/0031-9422(96)00213-0
- Matsuo Y, Ishida R, Matsumoto T, et al. Yendolipin, a novel lipobetaine with an inhibitory activity toward morphogenesis in a foliaceous green alga Monostroma oxyspermum. Tetrahedron. 1997;53(3):869–876.10.1016/S0040-4020(96)01007-1
- Kim KY, Choi KS, Kurihara H, et al. β-Glucuronidase inhibitory activity of bromophenols purified from Grateloupia elliptica. Food Sci Biotechnol. 2008;17(5):1110–1114.
- Bar S, Kumar JN, Amar M, et al. Catalytic aerobic oxidation of alcohols using recoverable IAPNO α-hydrogen nitrioxyl radicals. ChemCatChem. 2015;7(7):1129–1134.10.1002/cctc.201402985
- Nasir M, Saeidnia S, Mashinchian-Moradi A, et al. Sterols from the red algae, Gracilaria Salicornia and Hypnea flagelliformis, from Persian Gulf. Pharmacogn Mag. 2011;7(26):97–100.
- Chen JL, Moghaddam MF, Gerwick WH. Gloiosiphones A and B, novel metabolites from the red marine alga Gloiosiphonia verticillaris. J Nat Prod. 1993;56(8):1205–1210.10.1021/np50098a001
- Bernart MW, Gerwick WH, Corcoran EE, et al. Laurencione, a heterocycle from the red alga Laurencia spectabilis. Phytochemistry. 1992;31(4):1273–1276.10.1016/0031-9422(92)80276-K
- Diez T, Cabezas JA. Properties of two molecular forms of β-glucuronidase from the mollusk Littorina littorea L. Eur J Biochem. 1979;93:301–311.10.1111/ejb.1979.93.issue-2
