Abstract
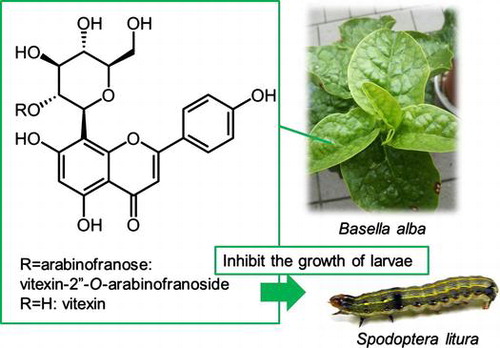
Basella alba is a perennial plant of the Basellaceae and is known by various common names including Malabar spinach. There are few insects that cause damage to B. alba. In this study, we examined the effect of B. alba leaves on the growth of Spodoptera litura larvae. B. alba leaves and a methanolic extract of the leaves inhibited the growth of S. litura larvae. Half of the larvae reared on the leaves died within 1 week. We found that two flavonoids, vitexin, and vitexin-2″-O-arabinofuranoside, were abundant in the methanol extract of leaves. When larvae were reared on purified vitexin or vitexin-2″-O-arabinofuranoside, their growth was significantly impaired compared with larvae reared on control spinach leaves. These results suggested that the flavonoid glycosides in B. alba leaves act as deterrents to S. litura larvae.
Flavonoid glycosides, vitexin and vitexin-2″-O-arabinofuranoside in B. alba leaves inhibit the growth of S. litura larvae.
To counter herbivore attack, plants produce secondary metabolites that have toxic, repellent, and/or antinutritional effects on herbivores. Plants produce defensive chemicals constitutively or in response to plant damage. These chemicals influence the feeding, growth, and survival of herbivores. Defensive compounds include simple phenylpropanoid derivatives, alkaloids, terpenes, and flavonoids. In addition, plants also release volatile organic compounds such as terpenes and nitrogen- and sulfur-containing compounds in response to damage by herbivores. These volatiles are involved in plant–insect interactions including plant defenses against insect herbivores. They can repel herbivores and attract natural enemies of the herbivores [Citation1]. Maize roots release (E)-β-caryophyllene, which attracts an entomopathogenic nematode, Heterorhabditis megidis, in response to feeding by larvae of the beetle Diabrotica virgifera virgifera [Citation2]. It was also reported that intercropping maize with molasses grass, Melinis minutiflora, decreased infestation by stem-borers in maize and increased larval parasitism by the parasitic wasp Cotesia sesamiae [Citation3]. Volatile agents produced by M. minutiflora repelled stem-borers and attracted C. sesamiae [Citation3].
Basella alba is a perennial plant of the Basellaceae and is known by various common names, including Malabar Spinach, Indian spinach, Ceylon spinach, and vine spinach. It is cultivated widely in many parts of the world. The leaves and stems are consumed as food and used in medicine. There are few insects that damage in B. alba. Intercropping celery and B. alba with cucumber significantly reduced whitefly numbers on cucumber [Citation4]. Geranylnitrile from B. alba has been reported to reduce whitefly colonization significantly [Citation4]. Whether B. alba has other chemical barriers to herbivores has not been investigated yet.
In this study, we examined how B. alba affects the growth of the Spodoptera litura. B. alba leaves and a methanolic extract of the leaves inhibited the growth of S. litura larvae. We found that vitexin and vitexin-2″-O-arabinofuranoside were abundant in the methanolic extract of B. alba. These flavonoid glycosides inhibited larval growth.
Materials and methods
Plants
B. alba nursery plants were purchased at a commercial nursery and planted in the experimental field at Agricultural Department of Yamagata University for insect bioassays. Spinach (Spinacia oleracea) leaves for bioassay and B. alba leaves for isolation of flavonoids were purchased from a local market in Tsuruoka, Japan. Leaves were washed thoroughly to remove the potential pesticide residues before the experiments.
Insect bioassay
Common cutworm, S. litura (Lepidoptera: Noctuidae) was purchased from Sumika Technoservice Co., Ltd. and reared on an artificial diet (Insecta-LFS, Nihon Nosan Kogyo Ltd., Yokohama) at 24 °C in a 16 h/8 h (light/dark) cycle. To determine the effect of B. alba leaves on the growth of S. litura larvae, 15 second instar larvae were placed in plastic Petri dishes with B. alba mature leaves for 2 weeks and weighed every day. Each larva was placed in a Petri dish with a leaf to feed freely. Spinach leaves were used as a control because spinach belongs to the same order as B. alba in the APG III system. We confirmed that vitexin and vitexin-2″-O-arabinofuranoside were not detected in the spinach leaves we used in the experiment by LC/MS.
Pieces of leaf (3 × 3 cm, ca. 500 mg) cut from spinach leaves were used to examine the effects of extracts and flavonoids of B. alba leaves on larvae. Two hundred microliters of the test solution was spread on the both side of leaf surface and allowed to air dry. Fifteen second instar larvae were placed in plastic Petri dishes with the leaves for a week and weighed every day. Leaves were changed every day.
Sample preparation for bioassay
Methanolic and aqueous extracts of B. alba leaves were prepared separately. For methanolic extracts, 500 mg of mature leaves were homogenized in 200 μL of methanol and centrifuged at 12,000 rpm for 5 min. The supernatant was evaporated to dryness and the residue was dissolved in 200 μL of ethanol. For the aqueous extract, 500 mg of mature leaves were homogenized in 200 μL of distilled water and centrifuged at 12,000 rpm for 5 min. The sample extract was applied on a piece of spinach leaf of almost the same weight as the B. alba leaf from which it was taken.
For flavonoid feeding experiments, vitexin or vitexin-2″-O-arabinofuranoside was dissolved in ethanol to produce a 500 ng/μL solution. We did not examine the dose-dependent effects of the flavonoids on larvae from quantitative constraints.
LC/MS analysis of methanolic extract of leaves
Leaves (500 mg) of B. alba were homogenized with 2 mL of MeOH and centrifuged for 5 min at 12,000 rpm. The supernatant was filtered through a 0.2 μm hydrophilic PTFE filter (Millipore). A 5-μL aliquot of each sample was injected into a Mightysil RP-18 GP column (50 × 2.0 mm, Kanto Chemical, Tokyo) and eluted using an Acquity system (Waters, Milford, MA). Solvent A was water containing 0.1% formic acid and solvent B was acetonitrile containing 0.1% formic acid (Kanto Chemical, Tokyo). The gradient was 0–3 min, 1% B, 3–12 min, linear gradient to 40% B, 12–13 min, linear gradient to 99% B, 13–17 min, 99% B, 17–18 min, linear gradient to 1% B, 18–22 min, 1% B. The flow rate was 0.2 mL/min and the column temperature was 40 °C. Mass spectra were recorded using a Synapt G2 HDMS (Waters, Milford, MA). MS acquisition was performed using the following conditions: Ionization mode, ESI positive mode; capillary voltage, 2.0 kV; cone voltage, 30 V; desolvation temperature, 550 °C; desolvation gas, 90 L/hour; source temperature 120 °C.
Structural determination of flavonoids by NMR
Fresh leaves (1 kg) of B. alba were extracted with 3L of MeOH. This methanolic extract was filtered and evaporated to dryness. The reside (9 g) was dissolved in 10% MeOH/H2O and subjected to reverse phase column (100 g; 100–200 mesh; Fuji Silysia Chemical) chromatography, eluting in sequence with MeOH/H2O (1:9 to 10:0). The pure compound 1 (120 mg) was obtained from the 40% MeOH fraction and compound 2 (25 mg) was obtained from the 60% MeOH fraction.
The 1H-NMR (600 MHz), 13C-NMR (150 MHz), and 2D-spectra of compound 1 in methanol-d4 and compound 2 in DMSO-d6 were recorded with TMS as an internal standard using a JEOL ECZ-600 spectrometer. Chemical shifts are given in δ values.
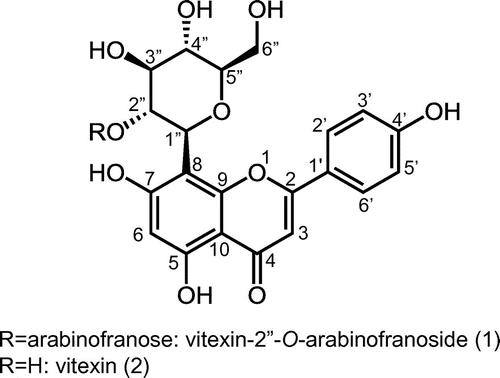
Compound 1 (5 mg) was hydrolyzed with 5% HCl/MeOH (1 mL) for 2 h at 65 °C. Vitexin was identified by 1H-NMR spectroscopy. The sugar arabinose was separated by TLC (TLC silica gel 60 F254, Merck) using acetone/n-BuOH/H2O as the eluent and identified by comparing the Rf value with the reference sample. Spots were detected by spraying with 10% vanillin in H2SO4 followed by heating.
Results
Effects of B. alba on the growth of larvae
The growth rates of S. litura larvae are shown in Figure . When larvae were reared on B. alba leaves, they initially ate the leaves aggressively. However, their consumption of leaves became infrequent within 1 or 2 days. Frass was not observed after 4 days, indicating that their feeding activity was reduced. The weights of larvae reared on B. alba leaves were significantly lower than those of larvae reared on control spinach leaves from day 3 to 6. Almost half of the larvae fed on B. alba leaves died within 1 week, and 93% died within 2 weeks. When larvae were kept unfed, their weight did not increase at all and they died within 3 days. These results suggested that B. alba contained compounds to deter larvae from feeding.
Figure 1. Effects of B. alba leaves on weight gain by S. litura larvae (mean ± SD, n = 13–15). An asterisk below the value indicates a significant difference from the control (spinach leaves) on the same day (p < 0.01 Student’s t-test).
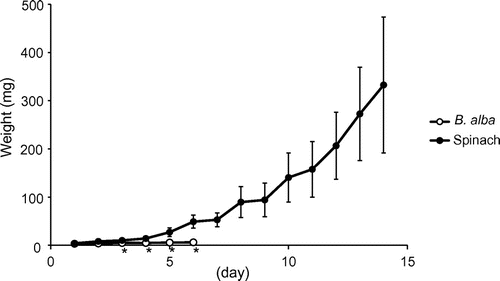
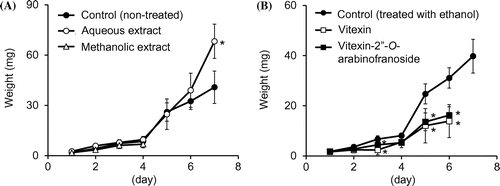
Figure (A) shows the effects of aqueous or methanolic extract effects of B. alba leaves on larvae. Eight of 15 larvae reared on the methanolic extract died in 5 days, and all of them died in 6 days. The absence of frass indicated their reduced feeding activities after 3 days. On the other hand, the weight gain of larvae reared on the leaves treated with aqueous extract was greater than that of larvae reared on non-treated leaves.
Figure 2. Effects of extracts (A) and flavonoids (B) of B. alba leaves on weight gain by S. litura larvae (mean ± SD, n = 13–15). The concentration of each flavonoid in a piece of leaf was 200 ng/mg. An asterisk next to the value indicates a significant difference from the control (non-treated or ethanol-treated leaves) on the same day (p < 0.01 Student’s t-test).
Identification of flavonoids in B. alba leaves
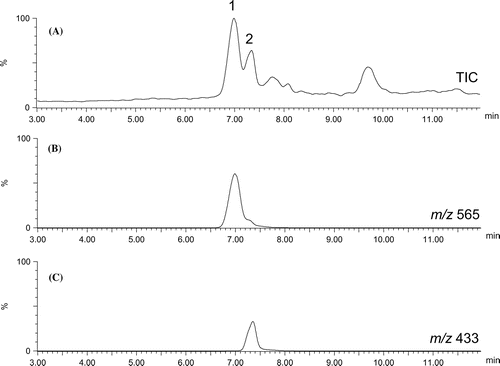
For chemical analysis of the methanolic extract of B. alba leaves, the extract was subjected to LC/MS. As shown in Figure , two distinct peaks were observed in the methanolic extract of B. alba leaves. These were characterized as flavonoid-hexoside-pentoside (1) and flavonoid-hexoside (2), respectively, from their fragment ion patterns in MS/MS experiments. Compound 1 (tR 7.0 min in Figure ) showed an [M + H]+ ion at m/z 565. MS/MS experiments on the ion at m/z 565 produced a product ion at m/z 433 after loss of a pentose sugar unit (neutral loss of 132 Da). We also detected product ions at m/z 313 and 283 due to the neutral loss of 120 and 150 Da. These product ions indicated the linkage of the hexose sugar to the aglycon was C-glucosidic [Citation5]. Compound 2 (tR 7.3 min) showed an [M + H]+ ion at m/z 433 and the MS/MS spectrum was similar to that of peak 1. Compounds 1 and 2 were isolated for further structural determination.
Figure 3. Typical TIC chromatogram of methanolic extract of B. alba leaves (A). (B) and (C) show extracted ion chromatograms for m/z 565 and 433, respectively.
Compound 2 was identified as vitexin (Figure ) by comparison of 1H- and 13C-NMR spectral data with an authentic sample purchased from Sigma-Aldrich (St. Louis, MO). Compound 1 was identified as vitexin-2″-O-arabinofuranoside (Figure ). 13C-NMR signals of 1 were in good agreement with those of vitexin-2″-O-arabinofuranoside reported by Palme et al. [Citation6]. Acid hydrolysis of 1 gave vitexin and arabinose.
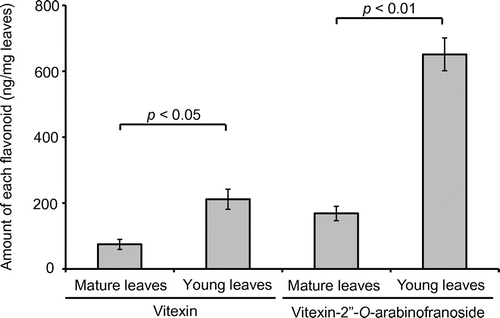
Because the levels of secondary metabolites were reported to be different between young and mature leaves [Citation7], the amounts of the flavonoids were measured in young and mature leaves, respectively. The amount of vitexin was calculated to be 74.9 ± 15.2 ng/mg in mature leaves and 211.8 ± 30.4 ng/mg in young expanding leaves by the peak area of its [M + H]+ ion (Figure ). The amount of vitexin-2″-O-arabinofuranoside was 168.6 ± 21.7 ng/mg in mature leaves and 651.5 ± 49.5 ng/mg in young leaves. Young leaves had higher concentrations of vitexin and vitexin-2″-O-arabinofuranoside than mature leaves.
Effects of flavonoids in B. alba leaves to larvae
We examined the effect of purified flavonoids from B. alba leaves on larvae (Figure (B)), because flavonoids are known to affect insect behavior and growth [Citation8]. When larvae were reared on spinach leaves containing approximately 200 ng/mg of vitexin or vitexin-2″-O-arabinofuranoside, their weight was significantly lower than those of the larvae reared on control spinach leaves at day 3 and from day 5 to 6. The tested flavonoid concentration (200 ng/mg) was determined from the concentration of the range between young and old B. alba leaves as shown in Figure . All of the larvae fed on vitexin or vitexin-2″-O-arabinofuranoside died within 1 week. Larvae initially ate leaves, but the absence of frass indicated impaired feeding activity after 5 days. These results confirmed that these flavonoids in B alba leaves act as deterrents to S. litura larvae.
Discussion
In this study, B. alba leaves inhibited the growth of S. litura larvae. Although the larvae reared on aqueous extracts of leaves gained more weight than larvae reared on non-treated leaves, methanolic extracts of the leaves inhibited larval growth. Aqueous extracts typically contain sugars and amino acids. It is reasonable to suggest that these nutrients in aqueous extracts could promote weight gain of the larvae. We identified two flavonoids, vitexin and vitexin-2″-O-arabinofuranoside, in methanolic extracts of leaves. When larvae were reared on leaves treated with these flavonoids, they ate the leaves for the first few days. Then, the larvae stopped eating and died. It was not clear whether the flavonoids were toxic to the larvae or if they just inhibit feeding behavior. Vitexin was not detected in aqueous extracts of B. alba leaves, but vitexin-2″-O-arabinofuranoside was detected in small amounts (18.5 ± 2.1 ng/mg). The concentration of vitexin-2″-O-arabinofuranoside in aqueous extracts would not be enough to inhibit the growth of larvae, or other substances in the aqueous extracts could interfere with the inhibition activity. Flavonoids often play roles in protecting plants from insect herbivory, although some insects have adapted to recognize the flavonoids present in their host plants. Wild species of peanuts were found to have high levels of flavonoids, which are related to resistance to S. litura [Citation9]. In rice, schaftoside, isoschaftoside, and neoschaftoside inhibit digestion in insects and they act as sucking deterrents to the brown plant hopper Niloparvata lugens [Citation10]. Dreyer et al. reported that vitexin has a deterrent activity on the green peach aphid, Myzus persicae and vitexin exhibited 50% inhibition in aphid feeding at a 0.1% concentration [Citation11]. We did not perform dose-dependent feeding inhibition experiments in this study, but our results showed that vitexin and vitexin-2″-O-arabinofuranoside in B. alba leaves inhibited the growth of S. litura larvae. To the best of our knowledge, this is the first report to suggest that vitexin and vitexin-2″-O-arabinofuranoside act as deterrents to a lepidopteran larva. Flavonoids can change the taste of the plants and reduce their nutritive value or act as toxins. Further studies are necessary to determine the detailed mechanism how vitexin and vitexin-2″-O-arabinofuranoside inhibit the growth of S. litura larvae.
Young leaves of B. alba had higher concentrations of vitexin and vitexin-2″-O-arabinofuranoside than mature leaves. This result indicated that young leaves have better chemical defenses than mature leaves. Young leaves of most plant species experience higher herbivore attack rates than mature leaves. It is suggested that plants could benefit by allocating more resources to defend vulnerable organs [Citation12]. Our results showed that the flavonoid glycosides in B. alba leaves form a barrier to defend the plant from herbivore attack as well as geranylnitrile to deter insects.
Author contribution
T. A. and T. M. designed the research. T. A. and S. I. performed the experiments with the aid of Y. S. and T. M. T. A. wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hare DJ. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol. 2011;56:161–180.10.1146/annurev-ento-120709-144753
- Rasmann S, Köllner TG, Degenhardt J, et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737.10.1038/nature03451
- Khan ZR, Ampong-Nyarko K, Chiliswa P, et al. Intercropping increases parasitism of pests. Nature. 1997;388:631–632.10.1038/41681
- Zhao Q, Zhu JJ, Qin Y, et al. Reducing whiteflies on cucumber using intercropping with less preferred vegetables. Entomol Exp Appl. 2014;150:19–27.10.1111/eea.2013.150.issue-1
- Prasain JK, Jones K, Kirk M, et al. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J Agric Food Chem. 2003;51:4213–4218.10.1021/jf030174a
- Palmea E, Biliaa AR, De Feo V, et al. Flavonoid glycosides from Cotoneaster thymaefolia. Phytochemistry. 1993;35:1381–1382.
- Masa CV, Díaz TS, Gallego JCA, et al. Quantitative variation of flavonoids and diterpenes in leaves and stems of Cistus ladanifer L. at different ages. Molecules. 2016;21:275.10.3390/molecules21030275
- Mierziak J, Kostyn K, Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecules. 2014;19:16240–16265.10.3390/molecules191016240
- Mallikarjuna N, Kranthi KR, Jadhav DR, et al. Influence of foliar chemical compounds on the development of Spodoptera litura (Fab.) in interspecific derivatives of groundnut. J Appl Entomol. 2004;128:321–328.10.1111/jen.2004.128.issue-5
- Harborne JB, Grayer RJ. Flavonoids and insects. In: Harborne JB, editor. The Flavonoids, Advances in Research since 1986. London: Chapman & Hall; 1994. p. 589–618.
- Dreyer DL, Jones KC. Feeding deterrency of flavonoids and related phenolics towards Schizaphis graminum and Myzus persicae: aphid feeding deterrents in wheat. Phytochemistry. 1981;20:2489–2493.10.1016/0031-9422(81)83078-6
- Rhoades DF. Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH, editors. Herbivores: their interaction with secondary plant metabolites. 1st ed. New York (NY): Academic Press; 1979. p. 1–54.