Abstract
Heavy metals in the environment are one of the major limiting factors affecting plant growth and development. However, the mechanisms of the heavy metal-induced physiological processes remain to be fully dissected. Here, we explored that SRO1 can physically interact with Glutathione Peroxidase 3 (GPX3) in Arabidopsis. Under Hg treatment, similar to the sro1, the growth of the gpx3/sro1 was repressed more seriously and the number of true leaves was more reduced and etiolated than that of the wild type and gpx3 plants. The electrolyte leakage rates showed that cell membrane integrity in gpx3/sro1 was damaged more severely than in the wild type and gpx3 mutant. The Real-time PCR results have shown that the expression of the APX1 and CAT3 was reduced under mercury stress in the sro1 and sro1/gpx3. Our results suggested that the combination of the SRO1 and GPX3 may be contributed to plant response to mercury stress by regulating ROS intracellular oxidative homeostasis.
The combination of the SRO1 and GPX3 has an important effect on plant tolerance of mercury stress.
The rapid development of an industrial economy worldwide has led to an increased concentration of heavy metals in the environment. Most of the heavy metals have no known nutritional function and are toxic to plants, which have become one of the major limiting factors affecting quality and production of crops [Citation1]. There are about 45 kinds of heavy metals, including As, Cd, Hg, Mn. And Hg is regarded as one of the most phytotoxic heavy metals. Exposure to these toxicities can causes stress in plants, including modification of protein profiles, the integrity of the cell membrane damaged, the induction of reactive oxygen species (ROS) production, cell death, decreased seed germination rates, inhibition of the photosynthesis, and transpiration [Citation2–4]. The plants have evolved a range of potential cellular mechanisms that may be involved in the detoxification of heavy metals and thus tolerance to metal stress, such as through the plasma membrane or other tissues to limit the absorption and transportation of heavy metal ions; through detoxification by a few small molecular compounds such as glutathione, oxalic acid, and citric acid salts; and through minimizing the effect of the heavy metal ion by transporting it into the vacuole for detoxification [Citation5–8]. In recent years, functional genomics technologies and protein analysis methods have been used to broaden our knowledge of the pathways that respond to heavy metal stress in plants. However, the detailed molecular mechanisms are still not clearly understood.
In plants, numerous signaling molecules and transcription factors have been demonstrated to play a regulatory role in adaptation of plants to excessive heavy metal mercury. For example, SRO1 (Similar to RCD One 1) belongs to a group of plant-specific proteins family with six members in Arabidopsis thaliana, including RCD1 (RADICAL-INDUCED CELL DEATH1), SRO1–SRO5 [Citation9], which have also been reported to play a potential role in Hg stress response. Excessive accumulation of reactive oxygen species (ROS) in sro1–1 mutants under Hg stress showed that SRO1-mediated heavy metal stress response may occur through the regulation of ROS homeostasis and metabolism [Citation10]. And some studies also have shown that heavy metal stress leads to the accumulation of high levels of hydrogen peroxide in plants [Citation11,12]. Cd induces oxidative stress by disturbing the balance between pro-oxidants, such as reactive oxygen species (ROS) and antioxidants [Citation13]. Cr stress can result in ROS generation through plasma membrane-located Rboh and elevated lipid peroxidation in rice [Citation14,15]. It was also found that the oxidative bursts induced by heavy metal are produced mainly through electron transport chains of mitochondria and chloroplasts and through peroxisomal activity [Citation16–18]. The regulation of heavy metal tolerance involves several enzymes, include Glutathione peroxidase (GPX), respiratory burst oxidase homolog (RBOH), superoxide Dismutase (SOD), catalase (CAT), and glutathione reductase (GR), which are essential for ROS homeostasis [Citation19]. During these stress, the cellular ROS balance is disturbed by either enhancing ROS generation or reducing ROS scavenging abilities.
As a large family of antioxidant enzymes, GPXs are a typical class of enzymes that scavenge ROS in organisms, which function similar to APX, CAT, and SOD [Citation20]. GPXs in animals have been demonstrated to be key and highly active enzymes involved in scavenging ROS [Citation21]. In yeast, it was found that the complex was formed between GPX and transcription factor YAP1 which is required for ROS sensing and scavenging [Citation22]. Some reports confirmed that the existence of plant GPXs also have enzymatic activity for scavenging ROS similar to animal GPXs, which plays an essential role in ROS homeostasis and stress signaling [Citation23,24]. However, the activities were lower than those of animals because they contain Cys at the putative catalytic site rather than the selenocysteine typical of animal GPXs [Citation25,26]. The plant GPX mRNA can be induced to increase with treatment of high salt and metal concentrations [Citation27]. It had been demonstrated that GPX3 in Arabidopsis might sense and transduce the H2O2 signal through coupled reaction both GPX3 and ABI2 redox state, thereby regulating plant response to ABA and drought stress [Citation28]. These studies revealed that the plant GPX can regulate many environmental stress responses and function coordinately in ROS scavenging and homeostasis.
In this study, we demonstrated that SRO1 could interact strongly with GPX3 through using molecular biology and biochemistry techniques. The investigation of the biological relevance for heavy metal mercury stress showed that the double mutants gpx3/sro1 were more sensitive to heavy metal stress than that of the single mutant gpx3 or sro1. And further studies have found that the expression of the ROS–scavenging genes was suppressed in single mutants, especially in double mutant. These results suggest that a conserved mechanism in plants is that SRO1 combination with GPX3 may orchestrate the cellular ROS generations and scavenging and homeostasis to modulate the plant response to heavy metal mercury stress.
Material and methods
Plant materials and growth conditions
The Arabidopsis thaliana ecotype Columbia (Col-0) was used in this study. The T-DNA insertion lines for SRO1 (At2g35510) Salk_126383 and GPX3 (AT2G43350) SALK_071176 were ordered from the Arabidopsis Biological Resource Center (ABRC) (http://www.arabidopsis.org/abrc/). The double mutants sro1/gpx3 were constructed by the single mutant sro1 crossing with gpx3, and the homozygous double mutant plants were screened by PCR amplification according to the method provided by the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu).
Surface-sterilized seeds were planted on 0.6% agar-containing MS medium (PhytoTechnology Laboratories) and kept for 3 days at 4 °C in the dark to break dormancy. The plates were then transferred to a culture room at 22 °C with a 16-h light/8-h dark photoperiod. For seedling growth, 7-day-old seedlings were transferred from the germination medium to 1.2% agar that was supplemented with various concentrations of HgCl2 treatments and placed vertically.
Yeast two-hybrid assays
Yeast two-hybrid interaction and protein pull-down assays were performed as described [Citation28]. The full-length coding region of SRO1 and GPX3 was respectively cloned in frame in the pACT2 and pAS2 vector to create the plasmid pACT2-SRO1 and pACT2-GPX3. Competent cells of S. cerevisiae strain Y190 were co-transformed simultaneously with pACT2-SRO1 and pAS-GPX3. Yeast cells co-transformed with pAS2 and pACT2 without inserts were used as negative controls.
GST pull-down assays
For GST pull-down assays, recombinant His-SRO1 and GST-GPX3 fusion proteins were respectively expressed in the pET-28a and pGEX-2TK vector. And the fusion proteins were respectively purified using the nickel–nitrilotriacetic acid agarose (Qiagen) and the glutathione resins (BD Biosciences) under native conditions according to the manufacturer’s protocol. Recombinant His-SRO1 and GPX3–GST extracts were incubated for 2 h at 4 °C together, and then the mixture was immobilized on glutathione resins. The resin was then washed extensively with the PBS buffer to remove unbound proteins. About 10 μL of the suspension was analyzed by western blotting using anti-His antibody.
Co-immunoprecipitation assays in protoplasts
For SRO1–GPX3 interaction assay in vivo, the fragments of the GPX3 and SRO1 coding region were amplified and respectively inserted into the pRT105-Flag and pRT105-Myc transient plasmid to produce the 35S: Flag-GPX3 and 35S:Myc-SRO1 recombinant vector. Myc-SRO1 was co-expressed with Flag-GPX3 in Arabidopsis protoplasts – prepared and transformed according to standard protocols [Citation29]. After an overnight incubation at 23 °C, the protoplasts were harvested and lysed. The total proteins were extracted from Arabidopsis protoplasts expressing 35S:GPX3-Flag and 35S:SRO1-Myc constructs and immunoprecipitated with Myc agarose beads. The samples were detected by anti-Flag and anti-Myc antibodies (Sigma-Aldrich).
Real-time PCR analysis
Total RNA was extracted from Arabidopsis 14-day-old Col-0 seedlings grown on MS medium using TRIzol reagent (Invitrogen). The RNA was treated with RNase-free DNase I (TaKaRa) to remove genomic DNA. Reverse transcription was done using 5 mg of total RNA and M-MLV reverse transcriptase (Promega) according to the manufacturer’s instructions. The resulting cDNA was diluted 10-fold and used as a template for the qRT-PCR amplification, which was performed with the primers listed in Supplemental Table 1. The qRT-PCR was carried out on the Stratagene Mx3005 QPCR system using SYBR Green to monitor double-stranded DNA products for 40 cycles, which was programmed for 40 cycles consisting of 95 °C for 15 s; 55 °C for 15 s; and 72 °C for 30 s. The relative expression level was calculated by 2−ΔΔCt and all the experiments were repeated three times. UBQ10 (AT4G05320) was the internal control. The relative expression levels were then normalized to that of the untreated control plant.
Electrolyte leakage measurements
The WT and mutant seedlings grown on 1.2% agar-containing MS solid medium for 15 days, with or without 200 mol/L of HgCl2 treatment, were used to measure the electrolyte leakage. The seedlings were taken into the test tube with 10 mL of distilled water and shaken at 100 rounds per minute for 12 h and the conductivity of solution (C1) was measured with LEICI DDSJ-308A conductivity meter, and then, the test tubes were heated at 100 °C for 2 h. After cooling to normal temperature, the total conductivity (CT) was measured. Electrolyte leakage rate was calculated as (C1/CT) × 100.
Statistical analysis
Each experiment was repeated three times with three independent replicates in the WT and mutants composed of atleast 10 plants for each. The error bars indicate standard deviations. Statistical analyses were carried out using SPSS software. Different letters indicate statistically significant differences by Tukey HSD (honestly significant difference) test (p < 0.05), which was applied for multiple comparisons.
Results
SRO1 can physically interact with GPX3 in vitro and in vivo
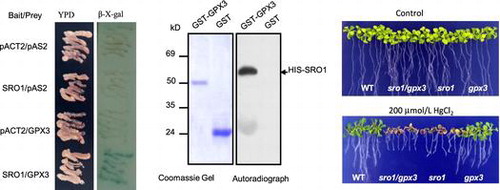
To further understand the mechanism of the SRO1 regulating the response to heavy metal mercury stress, we carried out yeast two-hybrid system to screen and identify proteins that interact with SRO1 full-length sequence. The CDS of SRO1 cloned in the pAS2 vector was used as bait to screen a prey library. Several unique SRO1 interaction partners were isolated, and subsequent DNA sequence analyzed and identified. One of them has been found to belong to the GPX protein family, which was the glutathione peroxidase 3 (Figure (A)). The colored β-galactosidase products appearing at 0.5 h, when the SRO1 bait was co-transformed with the GPX3 prey. As the negative control, the three combinations were almost completely ineffective at activating β-galactosidase reporter expression (Figure (B)). Yeast two-hybrid data were verified by in vitro pull-down assays using recombinant HIS-SRO1 protein and GST-fused GPX3 protein captured on GSH beads. This analysis (Figure (C)) confirmed the results of the yeast two-hybrid screens.
Figure 1. SRO1 interacts strongly with GPX3 in vitro and in vivo. (A) The interaction between the full-length SRO1 and the GPX3 in the yeast two-hybrid system. Yeast cells were transformed with pAS2-GPX3 as bait and pACT2-SRO1 as prey and were used for β-galactosidase filter assays. Empty bait and prey were used as control. (B) Quantitative analysis of β-galactosidase activity of the yeast strains in liquid culture showing the interaction between SRO1 and GPX3. Values are means of data from three independent experiments. Error bars indicate SD. (C) GST Pull-down assay. Contained His tag SRO1 was pull-down by GPX3-GST but not by GST. (D) Analysis of the interaction between SRO1 and GPX3 by co-immunoprecipitation in vivo. Combinations of Myc-SRO1 and Flag-GPX3, Flag-GPX1 were co-expressed in Arabidopsis protoplasts. Soluble extracts from protoplasts were analyzed using anti-Myc and anti-flag antibodies (Input). After immunoprecipitation with anti-Myc conjugated agarose, the immunoprecipitated products were detected with anti-Flag antibody to monitor GPX1 or GPX3 conjugates (Output).
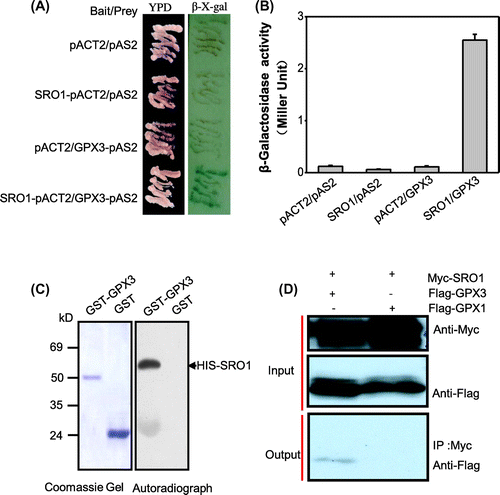
The interaction in vitro prompted us to investigate whether the SRO1 proteins can also interact with GPX3 in vivo, we monitored the association of transiently expressed SRO1 and GPX3 in protoplasts of Arabidopsis leaves using Co-immunoprecipitation. We generated 35S:6Myc-SRO1 and 35S:3Flag-GPX3 plasmid. The pair of plasmids was co-transformed into Arabidopsis protoplasts. Total proteins were extracted from the transformed protoplasts and subjected to immunoblots. Anti-cMyc antibody readily detected Myc-labeled SRO1 and anti-Flag antibody detected Flag-labeled GPX3 proteins in the transfected protoplasts. The SRO1 proteins were immunoprecipitated by anti-Myc and the GPX3 proteins were detected by the anti-flag antibodies. And more the signal was found in the protein co-transforming SRO1 and GPX3 compared with the negative control (Figure (D)). Together with the above results, we confirmed that SRO1 and GPX3 can interact strongly in vitro and in vivo.
SRO1 interaction with GPX3 is involved in Arabidopsis mercury stress resistance
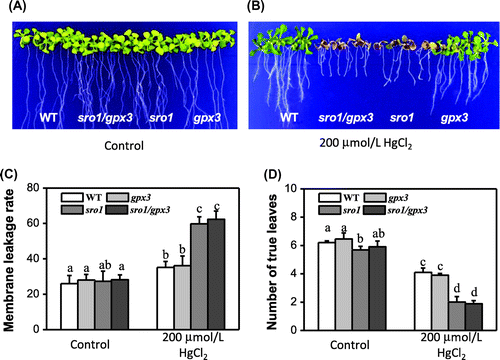
To assess whether the effect of SRO1 on heavy metal Hg stress tolerance is indeed mediated by interaction of GPX3, the double mutant sro1/gpx3 was created by crossing sro1 with gpx3 and screening the resulting F2 population for homozygosity at both loci (Figure S1). Growing after 15 days on MS medium, the seedlings of the double mutant sro1/gpx3 or single mutant sro1 and gpx3 grew similarly to that of the wild type (Figure (A)). When the seedlings were planted on MS medium containing HgCl2, both WT and gpx3 seedlings showed a slight inhibition in growth, whereas the sro1 mutant and the double mutant sro1/gpx3 plants were severely suppressed. And the double mutants sro1/gpx3 seem to be even more serious than the sro1 single mutant (Figure (B)), but the data by Turkey HSD test indicated that they have not significant difference (p = 0.068 > 0.05). With 200 μmol/L of HgCl2 treatment, sro1 mutant and the sro1/gpx3 double mutant plants began to appear albino and withered, which was consistent with the statistical analysis of the electrolyte leakage rate and the number of true leaves in all plants (Figure (C) and (D)). These results support a role for SRO1 interaction with GPX3 in protecting plants against heavy metal Hg stress.
Figure 2. Phenotypic analysis of WT, sro1, gpx3, and sro1/gpx3 double mutant with HgCl2 treatment. (A) All plants were grown for 15 days on MS medium under the same normal conditions as the controls. (B) Effects on the plant seedling response to 200 mmol/L HgCl2 stress. (C) Leakage of electrolytes from WT, sro1, gpx3, and sro1/gpx3 double mutant seedlings induced by 200 mmol/L HgCl2. (D) Number of true leaves from WT, sro1, gpx3, and sro1/gpx3 double mutant seedlings induced by 200 mmol/L HgCl2. (C) and (D)The average of three independent experiments is shown. Error bars indicate SD. Bars labeled by different letters are significantly different according to the Tukey HSD test for multiple comparisons (p < 0.05), and the same letters showed no significant difference from each other.
The expression of the ROS-scavenging enzymes was regulated by SRO1/GPX3 pathway in heavy metal stress
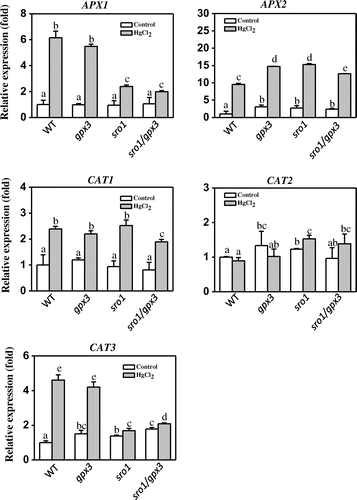
Our previous work indicated that SRO1 may regulate the response to Hg stress by scavenging excessive ROS and maintaining cellular redox homeostasis [Citation10]. To assess whether the ROS-scavenging enzymes is indeed involved in SRO1-GPX3 pathway regulating Hg stress tolerance, we therefore performed qRT-PCR to detect the expression levels of some ROS-scavenging enzymes in wild-type, sro1, gpx3, and sro1/gpx3. As shown in Figure , the expression of the gene APX1, CAT3 was reduced dramatically in sro1 and the double mutant sro1/gpx3 compared with its expression in WT and gpx3 with 200 μmol/L HgCl2 treatment. It has been shown that APX1 is an important component of the antioxidant defense system, and the APX1 expression was found to be induced significantly by Hg treatment [Citation30]. CAT3 transcription can be induced by mercury treatment and play an important role in controlling homeostasis of reactive oxygen species [Citation31,32]. Our results were consistent with these reports. Meanwhile, we also tested the other member transcription of the APX and CAT family, but no significant differences were found between WT and mutant seedlings. These data clearly indicate SRO1 interaction with GPX3 could modulate the expression of the APX1 and CAT3 to enhance plant heavy metal Hg stress tolerance.
Figure 3. Transcriptional levels of ROS scavenging enzyme in the wild type (WT), sro1, gpx3, and sro1/gpx3 double mutant plants with HgCl2 treatment. Total RNA was extracted from control and HgCl2-treated sro1, gpx3 and sro1/gpx3 seedlings. Expression of APX1, APX2, CAT1, CAT2, and CAT3 was assayed by qRT-PCR with gene specific primers. UBQ10 was used as the internal control. Relative expression levels were calculated by 2−ΔΔCt and normalized to the WT without HgCl2 treatment. Three independent qRT-PCR assays were performed and Error bars indicate SD. The analysis of variance was followed by the Tukey HSD test, and the bars with different letters are significantly different from each other at p < 0.05.
Discussion
Mercury pollution is one of the world’s most serious environmental problems. In plants, mercury stress can result in the accumulation of high levels of hydrogen peroxide, which is an important aspect of the harmfulness of heavy metal mercury to plant growth and development [Citation11]. Our previous report indicated that SRO1 regulated the mercury stress tolerance by sustaining ROS fine equilibrium between production and scavenging [Citation10]. However, the detailed mechanism of the process still needs to be explored. Here, we further found that GPX3 can physically interact with SRO1 in vitro or vivo, which may be as a partner to modulate the response to the mercury stress with SRO1 together. GPX3, one member of GPX family, plays a dual role within plants as a relay protein, first in the control of H2O2 homeostasis, and second in the transduction of an H2O2 signal in plant response to ABA and drought stress [Citation28]. These results implied SRO1 combination with GPX3 may contribute to plant response to mercury stress through sensing and keeping cellular redox homeostasis.
Upon exposure to mercury treatment, the sro1 mutant and the sro1/gpx3 double mutant showed increased sensitivity during vegetative growth, where the true leaves displayed an etiolated and bleached phenotype and the number of leaves was reduced compared with the WT and the gpx3 mutant (Figure ). The phenotype is consistent with the characteristics of plant growth under heavy metal stress [Citation33]. However, these mutants and WT have no difference in growth and development under the normal condition. Previous study showed that the ROS production induced by mercury was increased significantly in sro1 mutants, suggesting that loss of SRO1 combination with GPX3 may impair ROS homeostasis in plant. These genetic evidences further demonstrate that SRO1 and its partner GPX3 participate in the plant mercury tolerance together.
The excessive generation of ROS in cells will lead to disrupt ROS homeostasis and redox balance, driving cells into an oxidative state. And now, the balance of SOD, APX, and CAT activities will be crucial for suppressing cellular toxic ROS levels. In this study, we found that APX1 and CAT3 transcription in sro1 and sro1/gpx3 reduced significantly compared with the WT and gpx3 with mercury treatment, while the other member transcriptional level of APX and CAT family, all are ROS scavenging enzyme, was similar in WT and mutants. The fact that alterations of APX1 and CAT3 transcript levels are observed in sro1 and sro1/gpx3 suggests that GPX3 functions as an important partner of SRO1, not mainly scavenger, involving in plant response to mercury stress through orchestrating the cellular oxidative signaling and the expressional levels of the scavenging enzymes.
Author contributions
Xiaoliang Zhao is responsible for project planning and designed the study and wrote the paper. Lijie Gao, Pingning Jin, and Liusu Cui performed the experiments. Lijie Gao analyzed the data. All authors reviewed the results and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 31301165].
Supplemental data
The supplementary material for this paper is available online at https://doi.org/10.1080/09168451.2017.1408395.
Supplemental_Table.doc
Download MS Word (57 KB)Supplemantal_Figure.pptx
Download MS Power Point (107.5 KB)References
- Schutzendubel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot. 2002;53:1351–1365.
- Ouelhadj A, Kuschk P, Humbeck K. Heavy metal stress and leaf senescence induce the barley gene HvC2d1 encoding a calcium-dependent novel C2 domain-like protein. New Phytol. 2006;170:261–273.10.1111/nph.2006.170.issue-2
- Singh S, Parihar P, Singh R, et al. Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci. 2015;6:1143.
- Sugiyama M. Role of cellular antioxidants in metal-induced damage. Cell Biol Toxicol. 1994;10:1–22.10.1007/BF00757183
- Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 2006;88:1707–1719.10.1016/j.biochi.2006.07.003
- Haag-Kerwer A, Schafer HJ, Heiss S, et al. Cadmium exposure in Brassica juncea causes a decline in transpiration rate and leaf expansion without effect on photosynthesis. J Exp Bot. 1999;50:1827–1835.10.1093/jxb/50.341.1827
- Tuli R, Chakrabarty D, Trivedi PK, et al. Recent advances in arsenic accumulation and metabolism in rice. Mol Breeding. 2010;26:307–323.10.1007/s11032-010-9412-6
- Tian S, Xie R, Wang H, et al. Calcium deficiency triggers phloem remobilization of cadmium in a hyperaccumulating species. Plant Physiol. 2016;172:2300–2313.10.1104/pp.16.01348
- Jaspers P, Overmyer K, Wrzaczek M, et al. The RST and PARP-like domain containing SRO protein family: analysis of protein structure, function and conservation in land plants. BMC Genomics. 2010;11:170.10.1186/1471-2164-11-170
- Zhang X, Zhao X, Li B, et al. SRO1 regulates heavy metal mercury stress response in Arabidopsis thaliana. Chin Sci Bull. 2014;59:3134–3141.10.1007/s11434-014-0356-9
- Cho U, Park J. Mercury-induced oxidative stress in tomato seedlings. Plant Sci. 2000;156:1–9.10.1016/S0168-9452(00)00227-2
- Ortega-Villasante C, Hernández LE, Rellán-Álvarez R, et al. Rapid alteration of cellular redox homeostasis upon exposure to cadmium and mercury in alfalfa seedlings. New Phytol. 2007;176:96–107.10.1111/nph.2007.176.issue-1
- Xu J, Zhu Y, Ge Q, et al. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012;196:25–38.
- Panda SK. Chromium-mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. J Plant Physiol. 2007;164:1419–1428.10.1016/j.jplph.2007.01.012
- Trinh NN, Huang TL, Chi WC, et al. Chromium stress response effect on signal transduction and expression of signaling genes in rice. Physiol Plant. 2014;150:205–224.10.1111/ppl.2014.150.issue-2
- Dixit V, Pandey V, Shyam R. Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv. Azad) root mitochondria. Plant Cell Environ. 2002;25:687–693.10.1046/j.1365-3040.2002.00843.x
- Pandey V, Dixit V, Shyam R. Chromium effect on ROS generation and detoxification in pea (Pisum sativum) leaf chloroplasts. Protoplasma. 2009;236:85–95.10.1007/s00709-009-0061-8
- Steffens B. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front Plant Sci. 2014;5:685.
- Steffens B, Steffen-Heins A, Sauter M. Reactive oxygen species mediate growth and death in submerged plants. Front Plant Sci. 2013;4:179.
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875.10.1105/tpc.105.033589
- Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57:1825–1835.
- Delaunay A, Pflieger D, Barrault MB, et al. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481.10.1016/S0092-8674(02)01048-6
- Avsian-Kretchmer O, Gueta-Dahan Y, Lev-Yadun S, et al. The salt-stress signal transduction pathway that activates the gpx1 promoter is mediated by intracellular H2O2, different from the pathway induced by extracellular H2O2. Plant Physiol. 2004;135:1685–1696.10.1104/pp.104.041921
- Attacha S, Solbach D, Bela K, et al. Glutathione peroxidase-like enzymes cover five distinct cell compartments and membrane surfaces in Arabidopsis thaliana. Plant Cell Environ. 2017;40:1281–1295.10.1111/pce.v40.8
- Herbette S, Lenne C, Leblanc N, et al. Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur J Biochem. 2002;269:2414–2420.10.1046/j.1432-1033.2002.02905.x
- Herbette S, Roeckel-Drevet P, Drevet JR. Seleno-independent glutathione peroxidases. More than simple antioxidant scavengers. FEBS J. 2007;274:2163–2180.10.1111/ejb.2007.274.issue-9
- Ahmad P, Jaleel CA, Salem MA, et al. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. 2010;30:161–175.10.3109/07388550903524243
- Miao Y, Lv D, Wang P, et al. An arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell. 2006;18:2749–2766.10.1105/tpc.106.044230
- Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2:1565–1572.10.1038/nprot.2007.199
- Gomes-Junior RA, Moldes CA, Delite FS, et al. Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere. 2006;65:1330–1337.10.1016/j.chemosphere.2006.04.056
- Heidenreich B, Mayer K, Sandermann H, Ernst D. Mercury-induced genes in Arabidopsis thaliana: identification of induced genes upon long-term mercuric ion exposure. Plant Cell Environ. 2001;24:1227–1234.10.1046/j.0016-8025.2001.00775.x
- Du YY, Wang PC, Chen J, et al. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J Integr Plant Biol. 2008;50:1318–1326.10.1111/jipb.2008.50.issue-10
- Ortega-Villasante C, Rellán-Álvarez R, Del Campo FF, et al. Cellular damage induced by cadmium and mercury in Medicago sativa. J Exp Bot. 2005;56:2239–2251.10.1093/jxb/eri223
