Abstract
Cyclic 3′,5′-adenosine monophosphate (cAMP) phosphodiesterase (CPD) is an enzyme that catalyzes the hydrolysis of cAMP, a signaling molecule affecting diverse cellular and metabolic processes in bacteria. Some CPDs are also known to function in cAMP-independent manners, while their physiological roles remain largely unknown. Here, we investigated physiological roles of CPD in Shewanella oneidensis MR-1, a model environmental bacterium, and report that CPD is involved in amino-acid metabolism. We found that a CPD-deficient mutant of MR-1 (ΔcpdA) showed decreased expression of genes for the synthesis of methionine, S-adenosylmethionine, and histidine and required these three compounds to grow in minimal media. Interestingly, deletion of adenylate cyclases in ΔcpdA did not restore the ability to grow in minimal media, indicating that the amino acid requirements were not due to the accumulation of cAMP. These results suggest that CPD is involved in the regulation of amino acid metabolism in MR-1 in a cAMP-independent manner.
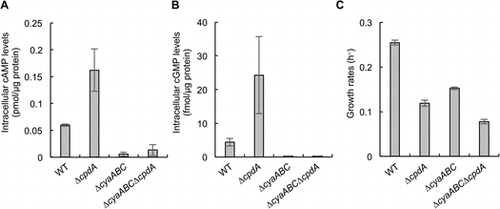
cAMP and cGMP levels and growth rates of cells aerobically grown in lactate minimal medium are shown in panels A, B, and C, respectively.
Abbreviations:
Members of the genus Shewanella belong to the class Gammaproteobacteria, and widely distributed in nature, including soil, sedimentary, marine, and freshwater environments [Citation1–3]. They are facultative anaerobes that can respire using a variety of electron acceptors, including oxygen, fumarate, nitrate, nitrite, dimethyl sulfoxide (DMSO), trimethylamine N-oxide, arsenate, and metal oxides [Citation2,4]. Shewanella oneidensis MR-1 is one of the most extensively studied strain in the genus Shewanella, and has also served as a model to study how environmental bacteria regulate multiple respiratory pathways [Citation5]. Previous studies have revealed that MR-1 regulates multiple anaerobic terminal reductase genes, including those for the reduction of metal oxides (omcA and mtrCAB), fumarate (fccA), and DMSO (dmsEFAB), using a cyclic 3′,5′-adenosine monophosphate (cAMP) receptor protein (CRP) [Citation6–8]. It is therefore suggested that intracellular cAMP concentration is a key factor in the regulation of anaerobic catabolic pathways in MR-1, although it remains largely unclear how this strain regulates the intracellular concentration of this signaling molecule [Citation9].
The cAMP/CRP-dependent regulatory system is conserved in many bacteria, and has been most extensively characterized in Escherichia coli in terms of its involvement in catabolite repression by glucose [Citation10,11]. Since cAMP is synthesized by adenylate cyclase (AC) and hydrolyzed by cAMP phosphodiesterase (CPD), intracellular cAMP levels are determined by relative activities of these enzymes. In E. coli, the activity of AC is known to be regulated depending on the intracellular glucose level by a post-translational mechanism in the phosphoenolpyruvate: sugar phosphotransferase system [Citation12,13]. In contrast, relatively little is known about the physiological roles and regulation of CPD.
Many studies have reported that the deletion or overexpression of genes encoding CPDs resulted in substantial phenotypic changes in E. coli and other bacteria [Citation14–18], indicating the importance of this enzyme for physiological regulation. For example, a cpdA-overexpressing strain of E. coli exhibited an enhanced resistance to oxidative stress [Citation16]. In Serratia marcescens, deletion of a CPD gene (cpdS) resulted in defective biofilm formation and reduced type I fimbriae production [Citation17]. Furthermore, Yin et al. [Citation18] recently reported that a cpdA-deletion mutant of S. oneidensis MR-1 exhibited impaired aerobic growth due to reduced expression of cytochrome bd and cbb3 oxidase genes. These phenotypes are all reportedly attributable to intracellular cAMP accumulation caused by the deletion of CPD, indicating that this enzyme is involved in the regulation of various cellular processes through its cAMP-hydrolyzing activity. However, a previous study has also reported that, although a cpdA-deletion mutant of Pseoudomonas aeruginosa exhibited a reduced growth rate in rich medium, this phenotype was not associated with elevated intracellular cAMP levels [Citation19]. It is therefore conceivable that CPD has as yet unknown functions that are independent of cAMP hydrolysis.
In the present study, we investigated the growth characteristics and transcriptome profiles of a CPD-deficient mutant of MR-1 in order to identify the physiological roles of CPD in this strain. The finding presented here suggest that CPD is involved in the regulation of amino acid metabolism in MR-1 via an unknown cAMP-independent mechanism.
Materials and methods
The bacterial strains, plasmids, and growth condition
The bacterial strains and plasmids used in this study are listed in Table . E. coli strains were cultivated in Luria-Bertani (LB) medium at 37 °C. The E. coli mating strain (WM6026) required 100 μg/ml 2,6-diaminopimelic acid for growth. S. oneidensis strains were cultured at 30 °C in LB or a lactate minimal medium (LMM) [Citation20] containing 10 mM lactate as the carbon and energy source. To examine the amino acid requirements of S. oneidensis strains, LMM was supplemented with 0.1 mM L-histidine, 0.1 mM L-methionine, and/or 35 μM S-adenosylmethionine (SAM). For aerobic cultivation, S. oneidensis strains were introduced into 300-mL baffled Erlenmeyer flasks containing 100 mL LB or 30-mL test tubes containing 5 mL LMM, and were cultivated with shaking on a rotary shaker at 180 rpm. For anaerobic cultivation, Shewanella strains were introduced into 13-mL test tubes containing 5 mL LMM supplemented with 30 mM lactate and 60 mM fumarate. Test tubes containing anaerobic cultures were capped with butyl rubber septa and polycarbonate screw caps, and purged with pure nitrogen gas. The optical densities at 600 nm (OD600) of the cultures were measured using UH5300 spectrophotometer (Hitachi, Tokyo) or miniphoto518R (Taitec, Tokyo). When necessary, 50 μg mL–1 kanamycin (Km) and 15 μg mL–1 gentamicin (Gm) were added to the culture media. Agar plates contained 1.6% Bacto agar (Difco, Franklin Lakes, NJ).
Table 1. Bacterial strains and plasmids used in this study.
To construct plasmid pBBRScpdA and pBBREcpdA, the S. oneidensis- and E. coli JM109-derived cpdA genes were amplified using Phusion High-Fidelity DNA polymerase (New England Biolabs, Beverly, MA,) and primer sets listed in Supplemental Table 1. The PCR products were digested by the restriction enzymes corresponding to the sites incorporated in the primers, and cloned between the corresponding sites of pBBR1MCS-5 [Citation21]. The resulting plasmids, pBBRScpdA and pBBREcpdA, were introduced into ΔcpdA cells by filter mating with E. coli WM6026.
Construction of gene-deletion strains
In-frame disruption of the cpdA, cyaA, cyaB and/or cyaC genes in MR-1 was carried out using a two steps homologous recombination method with plasmid pSMV-10, as described previously [Citation20,22,23]. Briefly, a 1.6-kb DNA fragment, consisting of upstream and downstream sequences of the cpdA, cyaA, cyaB, or cyaC genes joined by an 18-bp linker sequence, was constructed by PCR using the primers listed in Supplemental Table 1. The amplified DNA fragment was cloned into the SpeI site of pSMV-10. The resultant plasmid, pSMV-cpdA, pSMV-cyaA, pSMV-cyaB, or pSMV-cyaC, was introduced into MR-1 or its derivatives by filter mating with E. coli WM6026. Transconjugants (single-crossover clones) were selected on LB plates containing Km and were further cultivated for 20 h in LB medium lacking antibiotics. The cultures were spread onto LB plates containing 10% (w/v) sucrose to isolated Km-sensitive double-crossover mutants.
RNA extraction
Shewanella cells were cultured in LMM under aerobic condition, and were harvested at logarithmic growth phase (OD600 of 0.2 to 0.3). RNA was extracted using a Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. The extracted RNA was purified using a RNeasy Mini Kit and RNase-Free DNase Set (Qiagen, Valencia, CA). The quality of purified RNA was evaluated using an Agilent 2100 Bioanalyzer with RNA 6000 Pico reagents and RNA Pico Chips (Agilent Technologies, Santa Clara, CA) following the manufacturer’s instructions.
Determination of intracellular cAMP and cGMP concentrations
Shewanella cells were aerobically cultivated in LMM until stationary growth phase, and the cells collected from 1 mL of the cultures were suspended in 200 μL of lysis buffer containing 100 μM Ro20-1724, 500 μM 3-isobutyl-1-methylxanthine, 50 mM HEPES-NaOH (pH 7.4), and 5% TrionX-100, and were lysed for 30 min at room temperature. The cAMP and cyclic 3′,5′-guanosine monophosphate (cGMP) concentrations of the lysate were measured using a Cyclic AMP EIA Kit (Cayman Chemical, Ann Arbor, MI) and Cyclic GMP ELISA Kit (Cayman Chemical), respectively, according to the manufacturer’s instructions. The protein concentration of the lysate was measured using a Micro BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA). Concentrations of cAMP and cGMP were normalized based on protein contents.
Microarray analysis
Transcriptome analysis was performed using a custom DNA microarray for MR-1 (8 × 15K; Agilent Technologies) designed in a previous study [Citation24]. Cyanine 3 (Cy3)-labeled complementary RNA (cRNA) was synthesized from 50 ng total RNA using a Low Input Quick Amp WT Labeling Kit (Agilent Technologies) and purified using a RNeasy Mini Kit (Qiagen). For each array, 19 μL of the purified Cy3-labeled cRNA (600 ng) was mixed with 5 μL of 10× Blocking Agent, 1 μL of 25× Fragmentation Buffer, and 25 μL of 2× GE Hybridization Buffer HI-RPM. The resultant mixtures were hybridized to the arrays at 65 °C for 17 h. After hybridization, each microarray slide was washed with Gene Expression wash buffer 1 at room temperature for 1 min, followed by Gene Expression wash buffer 2 at 37 °C for 1 min. Slides were air dried for 1 min and scanned using an Agilent DNA Microarray Scanner at 5-μm resolution. Gene expression data (fluorescent intensities) were extracted from the scanned image using the Feature Extraction Software version 8.1 (Agilent Technologies) and normalized and statistically analyzed using GeneSpring GX version 11.5 (Agilent Technologies). The paired Student’s t-test and the Benjamini-Hochberg false discovery rate correction were used for statistical analysis. Differential expression for each probe was considered statistically significant when the fold change (FC) was ≥2.0 or ≤0.5 (|log2 FC| ≥ 1.0) at a P-value of <0.05. The microarray data have been deposited in the NCBI Gene Expression Omnibus (GEO) under the accession number GSE97220.
qRT-PCR
Quantitative reverse transcription-PCR (qRT-PCR) was performed using a LightCycler 1.5 instrument (Roche, Indianapolis, IN, USA) according to a method described previously [Citation25]. Briefly, a PCR reaction mixture contained 15 ng total RNA, 1.3 μL of 50 mM Mn(OAc)2 solution, 7.5 μL of LightCycler RNA Master SYBR Green I (Roche), and 0.15 μM primers listed in Supplemental Table 1. To generate standard curves, DNA fragments of target genes (pta, pflB, pykA, acs, hisC, metK, metR, metE, and 16S rRNA genes) were amplified by PCR using Ex Taq DNA polymerase (Takara, Tokyo) and the primer sets listed in Supplemental Table 1, and purified by gel electrophoresis using a QIAEX II Gel Extraction Kit (Qiagen) according to the manufacturer’s instructions. Standard curves were generated by amplifying a dilution series of the purified DNA fragments of each gene. Expression levels of target genes were normalized based on expression levels of the 16S rRNA gene.
Results and discussion
Growth characteristics of ΔcpdA
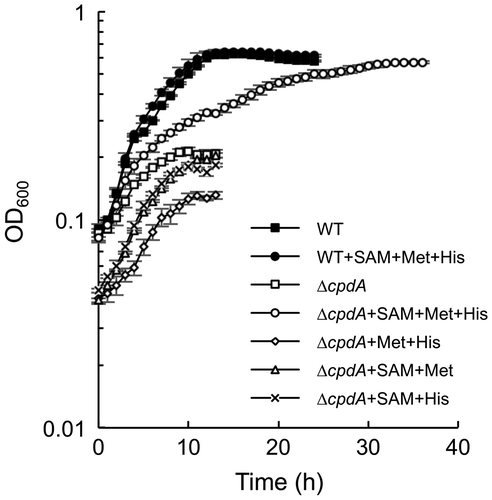
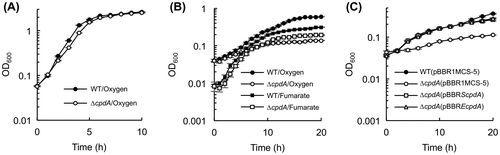
In S. oneidensis MR-1, CPD is encoded by the cpdA gene (SO_3901). To explore the physiological functions of CPD in MR-1, we constructed an in-frame deletion mutant of this gene (ΔcpdA), and compared the growth characteristics of ΔcpdA to those of the wild-type strain (WT). In this experiment, we cultivated these strains under aerobic and anaerobic conditions using the rich medium (LB medium) and the lactate minimal medium (LMM), since Yin et al. [Citation18] have reported that a ΔcpdA mutant of MR-1 exhibited a decreased growth rate when it was aerobically grown in LB medium, while the growth of this mutant in the minimal medium or under anaerobic conditions has not been characterized. When ΔcpdA was aerobically grown in rich medium (LB medium), the growth rate of this mutant (0.69 h–1) was slightly slower than that of WT (0.81 h–1; Figure (A)). This result is in good agreement with that reported by Yin et al. [Citation18]. The authors of that study concluded that the disruption of cpdA caused an elevated cAMP concentration, which repressed the expression of cytochrome oxidase genes and suppressed the cell growth under aerobic conditions. However, we found that ΔcpdA exhibited a more severe growth defect under aerobic conditions in LMM (Figure (B)). This growth deficiency was complemented in ΔcpdA transformed with a plasmid carrying the cpdA gene of MR-1 (pBBRScpdA) (Figure (C)). The ΔcpdA strain also showed impaired growth in LMM even when cells were cultivated under anaerobic fumarate-reducing conditions (Figure (B)). These observations suggest that the severe growth defect of ΔcpdA in LMM is not due to the reduced expression of cytochrome oxidase genes, but unknown mechanisms may have inhibited cell growth in the minimal medium. We also found that introduction of a plasmid expressing the cpdA gene of E. coli (pBBREcpdA) complemented the impaired growth of ΔcpdA in LMM (Figure (C)), suggesting that this growth deficiency is associated with a common function of bacterial CPDs.
Figure 1. Growth of S. oneidensis MR-1 and its derivatives in LB and LMM. Growth curves of cells cultured in LB under aerobic conditions are shown in panel A. Growth curves of cells cultured in LMM under aerobic and anaerobic fumarate-reducing conditions are shown in panels B and C, respectively. WT(pBBR1MCS-5), ΔcpdA(pBBR1MCS-5), ΔcpdA (pBBRScpdA), and ΔcpdA (pBBREcpdA) were aerobically grown in LMM containing Gm. Error bars represent standard deviations of means that were calculated from at least triplicate cultures.
Influence of intracellular cAMP and cGMP levels
To investigate whether the growth defect of ΔcpdA in LMM is associated with intracellular cAMP accumulation, we constructed deletion mutants of the AC genes (cyaA, cyaB, and cyaC) in the WT and ΔcpdA strains (ΔcyaABC and ΔcyaABCΔcpdA, respectively), and examined their intracellular cAMP levels and growth rates in LMM. Since previous studies have shown that CPD hydrolyzes cGMP as well as cAMP [Citation14,15], we also examined the accumulation of cGMP in the WT, ΔcpdA, ΔcyaABC, and ΔcyaABCΔcpdA cells (Figure ). In ΔcyaABC and ΔcyaABCΔcpdA, intracellular cAMP and cGMP concentrations were markedly lower than those in WT, while these compounds were accumulated in ΔcpdA at significantly high levels (Figure (A) and (B)). These results indicate that the cyaABC and cpdA genes encode functional ACs and CPD, respectively, and these enzymes are also involved in the synthesis and hydrolysis of cGMP, respectively. However, physiological roles of cGMP in bacteria remain largely unknown, although several studies have suggested the physiological significance of this molecule in bacteria [Citation15,26].
Figure 2. Intracellular cAMP and cGMP levels and growth rates of MR-1 and mutants. cAMP and cGMP levels and growth rates of cells aerobically grown in LMM are shown in panels A, B, and C, respectively. Error bars represent standard deviations of means that were calculated from at least triplicate experiments.
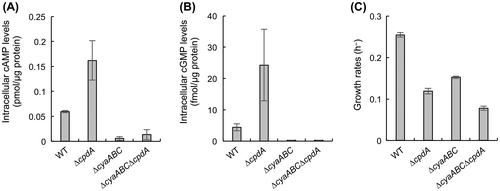
We next examined influences of the deletion of the AC genes on growth rates of ΔcpdA in LMM (Figure (C)). We found that the growth of ΔcyaABCΔcpdA was significantly slower than that of ΔcyaABC. Considering that intracellular cAMP and cGMP levels in ΔcyaABCΔcpdA were markedly lower than those in WT but comparable to those in ΔcyaABC, it is suggested that the accumulation of cAMP and/or cGMP is not the cause for the slow growth of ΔcpdA in LMM. The growth of ΔcyaABC and ΔcyaABCΔcpdA was slower than that of their parental strains (WT and ΔcpdA), and we consider that their grow retardation is related to the previously reported observation that the deletion of the cyaABC genes resulted in reduced expression of the cytochrome oxidase genes [Citation18].
Differentially expressed genes in ΔcpdA
To explore why ΔcpdA exhibited a severe growth defect in LMM, the transcriptome profiles of the ΔcpdA and WT cells aerobically grown in LMM were compared using DNA microarrays. The reliability of the microarray analysis was validated by qRT-PCR of 8 selected genes, and a high correlation (r2 = 0.99) was observed between the microarray and qRT-PCR results (Supplemental Figure 1).
The transcriptome analysis revealed that 442 genes were differentially expressed between WT and ΔcpdA with statistical significance of p < 0.05 and log2-fold change (FC) ≥ 1 or ≤–1. Among the 442 genes, 211 genes were up-regulated and 231 genes were down-regulated in ΔcpdA as compared to WT (Supplemental Tables 2 and 3). The up-regulated genes included the genes involved in anaerobic pyruvate metabolism (pflB) and acetate synthesis (pta and ackA). Under anaerobic conditions, MR-1 produces acetate as the main metabolite from lactate [Citation27] and synthesizes a substantial portion of ATP by substrate-level phosphorylation in the phosphotransacetylase-acetate kinase (Pta-AckA) pathway [Citation28]. PflB (pyruvate formate-lyase) catalyzes the conversion of pyruvate to acetyl-CoA and formate and plays the key role in anaerobic pyruvate degradation [Citation29]. A previous study has demonstrated that the deletion of crp or cyaC resulted in reduced expression of the pflB, pta, and ackA genes, indicating that the expression of these genes is affected by intracellular cAMP levels [Citation7]. We therefore consider that increased cAMP in ΔcpdA caused the up-regulation of these genes involved in anaerobic carbon metabolism.
Interestingly, the down-regulated genes included many genes involved in the synthesis of amino acids, including methionine, S-adenosylmethionine (SAM), and histidine (Table and Supplemental Table 3). In particular, expression of the metE and metH genes encoding methionine synthase was drastically decreased (18- to 315-fold) in ΔcpdA (Table ), suggesting that methionine synthesis is markedly inhibited in this mutant. Expression of the metK gene encoding SAM synthase was also significantly down-regulated in ΔcpdA (Table ). SAM serves as a major methyl donor that is utilized for various cellular processes, including the methylation of DNA and methyl-accepting chemotaxis proteins [Citation30], and a metK-deletion mutant of E. coli is reported to require exogenous SAM for growth [Citation31]. Expression of the gene cluster encoding enzymes involved in histidine synthesis (the his genes; SO_2067 to SO_2074) was also 3- to 9-fold decreased in ΔcpdA (Table ). In addition to these genes, the microarray analysis also detected the decreased expression of the genes involved in glutamate (glt), glutamine (gln), asparagine (asnB), tryptophan (trp), cysteine (cys), leucine (leu), isoleucine (ilv), proline (pro), glycine (glyA), and threonine (thr) in ΔcpdA. These results indicate that the deletion of cpdA has a global impact on amino acid metabolism in MR-1. We therefore hypothesized that the growth defect of ΔcpdA in LMM is attributable to the impaired expression of these amino acid-synthesis genes. Our analyses also suggest that there exists an unknown mechanism that globally regulates amino acid metabolism in MR-1, and CpdA is involved in it. Although Yin et al. [Citation18] reported that a cpdA-deletion mutant of MR-1 exhibited reduced expression of cytochrome bd and cbb3 oxidase genes, our microarray data did not reveal significant differences in the expression levels of these genes between WT and ΔcpdA (Supplemental Table 3). It is therefore likely that the expression of these genes is markedly affected by growth conditions, such as culture media and growth phase.
Table 2. Genes involved in methionine, SAM, and histidine synthesis with altered expression in ΔcpdA.
Fuchs et al. [Citation19] have reported that, among the phenotypes observed in a cpdA-deletion mutant of P. aeruginosa, reduced colony size and increased doubling time of cells grown in LB medium are independent of intracellular cAMP and cGMP levels. However, these phenotypes were not observed, when cells were grown in minimal media. They also demonstrated that the small-colony and slow-growth phenotypes were not restored, when the cpdA-deletion strain was complemented with plasmids carrying the active site-mutated derivatives of CpdA [Citation19]. From these results, Fuchs et al. [Citation19] suggest that an unknown compound(s) that can be hydrolyzed by this enzyme is involved in these phenotypes. Previous studies have also revealed that CPD can hydrolyze multiple phosphoester substrates, including cyclic 2′,3′-AMP [Citation15,32]. It is therefore possible to speculate that such an alternative substrate of CpdA serves as a global signaling molecule that controls the amino acid metabolism in MR-1.
Restored growth of ΔcpdA by adding amino acids
To test the hypothesis that the growth defect of ΔcpdA in LMM is due to the impaired ability to synthesize amino acids, we examined whether the addition of methionine, SAM, and histidine restored the growth defect of ΔcpdA in LMM (Figure ). We selected these compounds, since expression of the genes for their synthesis was severely repressed in this mutant (Table ). The results revealed that the addition of these tree amino acids markedly stimulated the growth of ΔcpdA in LMM, while it did not affect the growth of WT. No growth stimulation was observed in the absence of any one of these compounds (Figure ). These results confirm the idea that ΔcpdA impairs the ability to synthesize methionine, SAM, and histidine. However, the growth of ΔcpdA was not completely restored to the level of WT even when these three amino acids were supplemented. We consider that this is due to decreased abilities of ΔcpdA to synthesize the other amino acids, since the growth of ΔcpdA in rich medium was only slightly slower than that of WT (Figure (A)).
Conclusions
The present study demonstrates that deletion of cpdA causes reduced expression of many amino acid synthesis genes, including the met and his genes, thereby severely retarding the growth in minimal media. We also suggest that the growth defect of ΔcpdA is independent of intracellular cAMP and cGMP levels. It is therefore likely that CpdA is involved in an unknown mechanism that globally regulates amino acid metabolism in MR-1. Notably, the impaired growth of the ΔcpdA mutant of MR-1 was complemented by the cpdA genes derived from E. coli (Figure (C)), suggesting that the physiological roles of CpdA found in the present study is attributable to a common activity of CPDs in different bacterial species. We expect that further investigation of the catalytic activity and physiological functions of this enzyme will reveal as yet unexplored signal transduction mechanisms in bacteria.
Author contributions
TK carried out the majority of the experimental work and drafted the manuscript. AK conceived of the study, participated in its design and coordination, and drafted the manuscript. KW supervised the study and performed manuscript editing. All authors read and approved the final manuscript.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) Fellows to TK [grant number 16J08653]; Young Scientists (B) to AK [grant Number 26850056].
Supplemental data
Supplemental data for this article can be accessed [https://doi.org/10.1080/09168451.2017.1413326].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary_Table_3.xlsx
Download MS Excel (35.6 KB)Supplementary_Table_2.xlsx
Download MS Excel (35.3 KB)Supplementary_Table_1.docx
Download MS Word (122.8 KB)171014kasai_cpdA_FigS1.pdf
Download PDF (62.2 KB)References
- Venkateswaran K, Moser DP, Dollhopf ME, et al. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol. 1999;49(2):705–724.10.1099/00207713-49-2-705
- Fredrickson JK, Romine MF, Beliaev AS, et al. Towards environmental systems biology of Shewanella. Nat Rev Microbiol. 2008;6(8):592–603.10.1038/nrmicro1947
- Hau HH, Gralnick JA. Ecology and biotechnology of the genus Shewanella. Annu Rev Microbiol. 2007;61:237–258.10.1146/annurev.micro.61.080706.093257
- Myers CR, Nealson KH. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988;240(4857):1319–1321.10.1126/science.240.4857.1319
- Sturm G, Richter K, Doetsch A, et al. A dynamic periplasmic electron transfer network enables respiratory flexibility beyond a thermodynamic regulatory regime. ISME J. 2015;9(8):1802–1811.10.1038/ismej.2014.264
- Saffarini DA, Schultz R, Beliaev A. Involvement of cyclic AMP (cAMP) and cAMP receptor protein in anaerobic respiration of Shewanella oneidensis. J Bacteriol. 2003;185(12):3668–3671.10.1128/JB.185.12.3668-3671.2003
- Charania MA, Brockman KL, Zhang Y, et al. Involvement of a membrane-bound class III adenylate cyclase in regulation of anaerobic respiration in Shewanella oneidensis MR-1. J Bacteriol. 2009;191(13):4298–4306.10.1128/JB.01829-08
- Kasai T, Kouzuma A, Nojiri H, et al. Transcriptional mechanisms for differential expression of outer membrane cytochrome genes omcA and mtrC in Shewanella oneidensis MR-1. BMC Microbiol. 2015;15(1):1319.
- Kouzuma A, Kasai T, Hirose A, et al. Catabolic and regulatory systems in Shewanella oneidensis MR-1 involved in electricity generation in microbial fuel cells. Front Microbiol. 2015;6:609.
- Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56(1):100–122.
- Kolb A, Busby S, Buc H, et al. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–797.10.1146/annurev.bi.62.070193.003533
- Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70(4):939–1031.10.1128/MMBR.00024-06
- Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol. 2008;11:87–93.10.1016/j.mib.2008.02.007
- Imamura R, Yamanaka K, Ogura T, et al. Identification of the cpdA gene encoding cyclic 3′,5′-adenosine monophosphate phosphodiesterase in Escherichia coli. J Biol Chem. 1996;271(41):25423–25429.10.1074/jbc.271.41.25423
- Matange N. Revisiting bacterial cyclic nucleotide phosphodiesterases: cyclic AMP hydrolysis and beyond. FEMS Microbiol Lett. 2015;362(22):1–9.
- Barth E, Gora KV, Gebendorfer KM, et al. Interplay of cellular cAMP levels, S activity and oxidative stress resistance in Escherichia coli. Microbiology. 2009;155(5):1680–1689.10.1099/mic.0.026021-0
- Kalivoda EJ, Brothers KM, Stella NA, et al. Bacterial cyclic AMP-phosphodiesterase activity coordinates biofilm formation. PLoS ONE. 2013;8(7):e71267.10.1371/journal.pone.0071267
- Yin J, Meng Q, Fu H, et al. Reduced expression of cytochrome oxidases largely explains cAMP inhibition of aerobic growth in Shewanella oneidensis. Sci Rep. 2016;6:27.10.1038/srep24449
- Fuchs EL, Brutinel ED, Klem ER, et al. In vitro and in vivo characterization of the Pseudomonas aeruginosa cyclic AMP (cAMP) phosphodiesterase CpdA, required for cAMP homeostasis and virulence factor regulation. J Bacteriol. 2010;192(11):2779–2790.10.1128/JB.00168-10
- Kouzuma A, Meng X-Y, Kimura N, et al. Disruption of the putative cell surface polysaccharide biosynthesis gene SO3177 in Shewanella oneidensis MR-1 enhances adhesion to electrodes and current generation in microbial fuel cells. Appl Environ Microbiol. 2010;76(13):4151–4157.10.1128/AEM.00117-10
- Kovach ME, Elzer PH, Hill DS, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166(1):175–176.10.1016/0378-1119(95)00584-1
- Saltikov CW, Newman DK. Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci USA. 2003;100(19):10983–10988.10.1073/pnas.1834303100
- Newton GJ, Mori S, Nakamura R, et al. Analyses of current-generating mechanisms of Shewanella loihica PV-4 and Shewanella oneidensis MR-1 in microbial fuel cells. Appl Environ Microbiol. 2009;75(24):7674–7681.10.1128/AEM.01142-09
- Kouzuma A, Oba H, Tajima N, et al. Electrochemical selection and characterization of a high current-generating Shewanella oneidensis mutant with altered cell-surface morphology and biofilm-related gene expression. BMC Microbiol. 2014;14(1):190.10.1186/1471-2180-14-190
- Kouzuma A, Hashimoto K, Watanabe K. Influences of aerobic respiration on current generation by Shewanella oneidensis MR-1 in single-chamber microbial fuel cells. Biosci Biotechnol Biochem. 2012;76(2):270–275.10.1271/bbb.110633
- Gomelsky M. cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all!. Mol Microbiol. 2011;79(3):562–565.10.1111/j.1365-2958.2010.07514.x
- Tang YJ, Hwang JS, Wemmer DE, et al. Shewanella oneidensis MR-1 fluxome under various oxygen conditions. Appl Environ Microbiol. 2007;73(3):718–729.10.1128/AEM.01532-06
- Hunt KA, Flynn JM, Naranjo B, et al. Substrate-level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1. J Bacteriol. 2010;192(13):3345–3351.
- Pinchuk GE, Geydebrekht OV, Hill EA, et al. Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation, and fumarate respiration conditions. Appl Environ Microbiol. 2011;77(23):8234–8240.10.1128/AEM.05382-11
- Chiang PK, Gordon RK, Tal J, et al. S-Adenosylmethionine and methylation. FASEB J. 1996;10(4):471–480.
- Driskell LO, Tucker AM, Winkler HH, et al. Rickettsial metK-encoded methionine adenosyltransferase expression in an Escherichia coli metK deletion strain. J Bacteriol. 2005;187(16):5719–5722.10.1128/JB.187.16.5719-5722.2005
- Keppetipola N, Shuman S. A phosphate-binding histidine of binuclear metallophosphodiesterase enzymes is a determinant of 2′,3′-cyclic nucleotide phosphodiesterase activity. J Biol Chem. 2008;283(45):30942–30949.10.1074/jbc.M805064200