Abstract
Isoprenoids play widely differing roles in various physiological processes in animals and plants. Geranylgeraniol (GGOH) is an isoprenoid found in plants, and is an important metabolic derivative in the isoprenoid/cholesterol synthesis pathway. Earlier studies focused on GGOH’s ability to improve the side effects of bisphosphonate therapy by regulating the mevalonate pathway. More recently, the mevalonate pathway-independent effects of GGOH have been described, including anti-inflammatory, anti-tumorigenic, and neuroprotective activities. It is noteworthy that GGOH regulates the steroidogenesis pathway in testis-derived I-10 tumor cells. Testosterone is a hormone produced via steroidogenesis in testicles and plays a role in fetal development and the male reproductive system. GGOH enhanced testosterone and progesterone (its precursor) levels in I-10 cells by activating adenylate cyclase via cAMP/PKA signaling, without altering phosphodiesterase activity. These findings highlight the potential benefits of GGOH as a therapeutic agent for low testosterone levels, such as late-onset hypogonadism in men.
Geranylgeraniol enhances testosterone production.
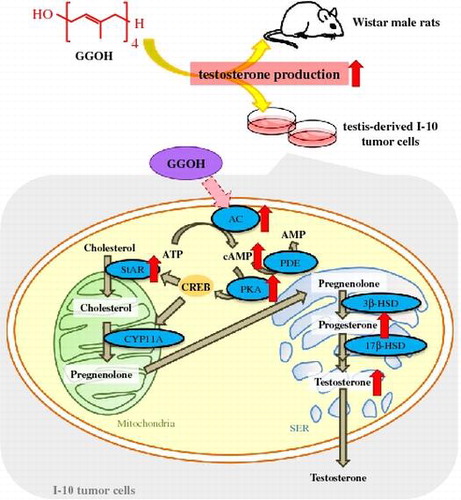
Isoprenoids (also referred to as terpenoids) are the largest and most structurally diverse family consisting of isoprene (C5) units in nature, with approximately 55,000 compounds [Citation1], and classified based on the number of isoprene units. The major groups are monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), and triterpenes (C30). As far as we know, isoprenoids play numerous functional roles in the physiological processes that are required for cell growth in plants and animals [Citation2–4], and are currently utilized as commercial flavors, fragrances, dietary supplements, and even as anticancer drugs [Citation5,6]. Two distinct and independent isoprenoid biosynthetic pathways occur in nature. They both synthesize isopentenyl diphosphate and its isomer dimethylallyl diphosphate, which are the central intermediate of isoprenoids [Citation7]. Although certain specialized isoprenoids are cataloged with well-characterized biochemical functions (e.g. sterols, carotenoids, and quinones), the functions of numerous other isoprenoids are still unknown [Citation8] Diterpenes geranylgeraniol (GGOH) can be ingested from vegetables and grains, including rice. Geranylgeranyl diphosphate (GGPP) may also be hydrolyzed into GGOH by intestinal phosphatase [Citation9] and then absorbed into the body. In the present article, we highlight the effects of exogenous GGOH on testosterone production.
Current knowledge on the potential benefits of GGOH
GGOH is an intermediate in the biosynthesis of vitamins E and K, as well as an important metabolic derivative in the isoprenoid/cholesterol synthesis pathway. Most previous studies on the effects of GGOH have focused on the mevalonate pathway (MVP), showing that GGOH improves the side effects of bisphosphonate therapy by regulating the MVP and inhibiting osteoclast formation [Citation10–17] Other studies have found that GGOH restores the function of cells which were treated with statin or are deficient in mevalonate kinase [Citation18–20], maintains the beneficial effects of cholesterol synthesis in human THP-1 cells treated with a MVP inhibitor [Citation21], and exerts endotoxin tolerance in statin-treated murine macrophages [Citation22]. These also suggest that GGOH can prevent the cytotoxicity of statin treatments by regulating MVP. Recent studies, however, indicate that GGOH also possesses beneficial effects independently from the regulation of the MVP. These include antibacterial [Citation23], anti-inflammatory [Citation24,25], anti-tumorigenic [Citation26–29], and neuroprotective activities [Citation30,31]. According to these results, GGOH is required for the protein isoprenylation that controls cytoskeletal reorganization, vesicular fusion, and further regulates apoptosis and cell survival. We were the first to report that GGOH enhanced testosterone levels in murine Leydig tumor cells [Citation32]. These findings highlighted the need for further research into the potential benefits of GGOH as a local therapeutic agent in several diseases.
Steroidogenesis in testicular Leydig cells
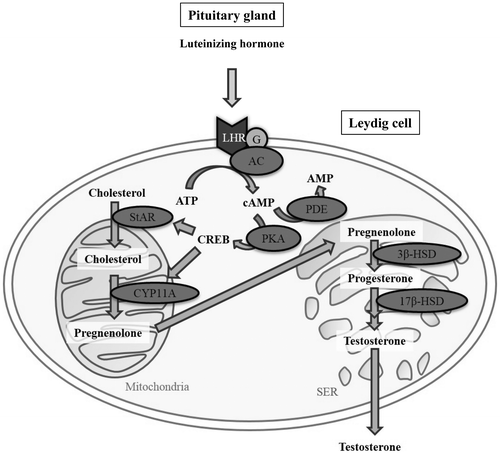
As shown in Figure , luteinizing hormone (LH) from pituitary gland regulates testosterone production in testicular Leydig cells via the LH receptor, which then promotes the 3′,5′-cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway by modulating G proteins. Intracellular cAMP promotes the cAMP/PKA pathway, which is mediated by the activities of adenylate cyclases (ACs) and phosphodiesterases (PDEs). The elevation of cAMP regulates the activation of PKA to stimulate signaling in downstream steroidogenic proteins. Steroidogenic acute regulatory protein (StAR) regulates cholesterol transfer within the mitochondria in a rate-limiting step to initiate steroidogenesis in Leydig cells. Moreover, a cholesterol side-chain cleavage enzyme, cytochrome P450scc (also referred to as CYP11A), catalyzes a cascade of steroidogenic reactions that convert cholesterol to pregnenolone, the metabolic intermediate in the biosynthesis of most of the steroid hormones including testosterone. Testosterone inhibits the release of LH from the pituitary gland by negative feedback via the hypothalamus [Citation33,34].
Figure 1. Steroidogenic pathway in testicular Leydig cells. AC, adenylate cyclase; CREB, cAMP response element-binding protein; CYP11A, cholesterol side-chain cleavage enzyme; G, G protein; LH, luteinizing hormone; LHR, LH receptor; Mito, mitochondria; PDE, phosphodiesterase; PKA, protein kinase A; SER, smooth endoplasmic reticulum; StAR, steroidogenic acute regulatory protein.
Testosterone level as a predictor of various diseases in men
Testosterone is crucial for the development of fetuses and male reproductive systems, as well as for sexual function. Increasing evidence shows that testosterone deficiencies cause infertility and sexual dysfunction in men, sometimes resulting in late-onset hypogonadism (LOH) [Citation35–37]. In an aging population with LOH, the common symptoms include sexual dysfunction, muscle weakness and depression. It is noteworthy that low testosterone levels may predict the development of several diseases, such as type 2 diabetes mellitus and cardiovascular diseases [Citation38–41]. The current treatment for LOH is testosterone replacement therapy. Although it may improve bodily health, quality of life, and sexual function in men, it can also increase mortality [Citation42–44]. In our recent study, it was revealed that GGOH may boost testosterone levels in Leydig cells [Citation32].
GGOH stimulates testosterone and progesterone production in testis-derived Leydig cells
The following important turning points in understanding the connection between GGOH and testosterone were previously reported by us. First, we found that menaquinone-4 (MK-4), one of vitamin K2, stimulated testosterone production in I-10 cells derived from a Leydig cell testicular tumor in a mouse [Citation45]. Second, we found that both MK-4 and GGOH showed anti-inflammatory effect in a lipopolysaccharide-induced inflammation model via inhibition of nuclear factor-κB activation [Citation25,46]. According to these results, we presumed that the geranylgeranyl side chain of MK-4 was important in expressing this activity and, subsequently, investigated the effects of GGOH on testosterone production [Citation32].
GGOH enhanced the levels of testosterone and its precursor, progesterone, in I-10 cells in a dose- and time-dependent manner (as shown in Figure ). The testosterone-enhancing abilities of other structurally related isoprenoids (geraniol, GOH, 2 isoprene units; and farnesol, FOH, 3 isoprene units), as well as those of chemicals structurally close to GGOH (phytol and GGPP), were also examined. Testosterone and progesterone levels in I-10 cell culture mediums markedly increased in the presence of phytol and GGPP, but not in the presence of GOH. Meanwhile, FOH enhanced progesterone, but not testosterone levels. These results indicated that phytol and GGOH have similar effects on testosterone and progesterone production although, unexpectedly, GOH did not affect steroid production in I-10 cells. Recent researches have revealed the biological activity of compounds derived from the isoprenoid/cholesterol synthesis pathway. These isoprenoids, including FOH, GOH, and phytol, regulate various biological processes [Citation47–49]. The results indicated that not only GGOH, but other isoprenoid derivatives, can enhance testosterone and progesterone levels, although the mechanisms by which they exert these effects are yet to be clarified.
Figure 2. Testosterone and progesterone levels after GGOH treatment in I-10 cells. Cells were treated with of GGOH for 0–24 h, and testosterone (A) and progesterone (B, C) levels in cultured medium were measured. Data are presented as mean ± SD (n = 3). *p < 0.05, **p < 0.01 vs. 0 μM. Different letters indicate significant differences (p < 0.05). Data for panels A–C were taken from reference [Citation32].
![Figure 2. Testosterone and progesterone levels after GGOH treatment in I-10 cells. Cells were treated with of GGOH for 0–24 h, and testosterone (A) and progesterone (B, C) levels in cultured medium were measured. Data are presented as mean ± SD (n = 3). *p < 0.05, **p < 0.01 vs. 0 μM. Different letters indicate significant differences (p < 0.05). Data for panels A–C were taken from reference [Citation32].](/cms/asset/0e57f7cd-dd0b-433f-9b91-fdab402a1629/tbbb_a_1415129_f0002_b.gif)
GGOH regulates the cAMP/PKA signaling pathway by modulating AC activity
Our findings indicated that GGOH could enhance progesterone and testosterone production via the regulation of PKA activity in a cAMP-dependent pathway (Figure (A) and (B)). Moreover, as shown in Figure (C) and (D), the enhancement of progesterone production in GGOH supplementation was suppressed by AC inhibitor, MDL-12,330A (MDL); on the other hand, progesterone level did not rise significantly with GGOH and addition of PDE inhibitor, 3-isobutyl-1-methylxanthine (IBMX). These results suggested that GGOH regulates cAMP levels by inducing AC activity, and not by suppressing PDE. To further confirm that GGOH stimulates steroidogenesis via the induction of AC activity, we downregulated AC mRNA expression using small interfering RNA. The results met our expectations, indicating that GGOH stimulated steroidogenesis via the regulation of AC, especially the activation of AC9 [Citation32]. There are 10 types of AC isoforms (AC1–10) in mammals; AC1 to AC9 are transmembrane enzymes, while AC10 is soluble. The transmembrane isoforms are mainly regulated via G-protein-coupled receptors [Citation50,51]. Human AC9 mRNA is abundant in the brain, heart, and skeletal muscle [Citation52]. AC9 is highly expressed in I-10 cells and our findings by using AC9 siRNA (Figure (E)) showed that AC9 may regulate cAMP levels after GGOH stimulation. However, among the gene expression of steroidogenesis-related proteins, both the mRNA and protein levels of StAR increased in the presence of GGOH. StAR functions in the rate-limiting step of steroid production in Leydig cells and is required for the transport of cholesterol to the inner mitochondrial membrane [Citation53]. We showed that GGOH treatment increases the expression of StAR, which acts downstream in the cAMP/PKA pathway. Taken together, our results demonstrate that GGOH enhances testosterone and progesterone production in I-10 cells via the induction of cAMP/PKA signaling (Figure ).
Figure 3. GGOH regulates cAMP/PKA signaling pathway by modulating AC activity in I-10 cells. (A) Intracellular cAMP levels were enhanced after GGOH treatment (30 μM) in I-10. (B–D) GGOH (30 μM) stimulated cAMP/PKA signaling pathway via regulation of AC but not PDE activity in I-10 cells. H89: PKA inhibitor, MDL: AC inhibitor, IBMX: PDE inhibitor. (E) Enhanced progesterone production by GGOH (30 μM) was diminished by AC9 siRNA treatment. Data are presented as mean ± SD (n = 3). **p < 0.01 vs. Ctrl group. Different letters indicate significant differences (p < 0.05). Data for panels A–D were reproduced from reference [Citation32].
![Figure 3. GGOH regulates cAMP/PKA signaling pathway by modulating AC activity in I-10 cells. (A) Intracellular cAMP levels were enhanced after GGOH treatment (30 μM) in I-10. (B–D) GGOH (30 μM) stimulated cAMP/PKA signaling pathway via regulation of AC but not PDE activity in I-10 cells. H89: PKA inhibitor, MDL: AC inhibitor, IBMX: PDE inhibitor. (E) Enhanced progesterone production by GGOH (30 μM) was diminished by AC9 siRNA treatment. Data are presented as mean ± SD (n = 3). **p < 0.01 vs. Ctrl group. Different letters indicate significant differences (p < 0.05). Data for panels A–D were reproduced from reference [Citation32].](/cms/asset/bdc6b171-ac09-4b59-a602-bb271f9e637a/tbbb_a_1415129_f0003_b.gif)
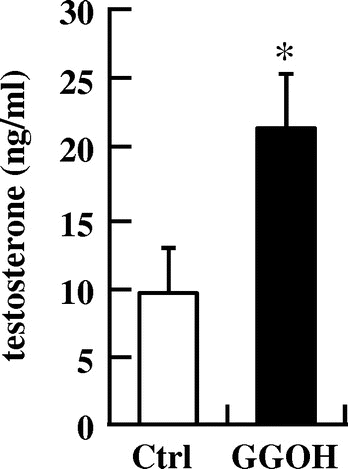
Dietary supplementation of GGOH enhances plasma testosterone levels in rats
We also found that dietary supplementation of MK-4 enhanced plasma testosterone levels in rats, without any alternation of plasma LH levels [Citation45]. Based on the results of our cell-based experiments [Citation32], we conducted further experiments on the effects of GGOH on testosterone production in animals. Eight-week-old Wistar male rats were purchased from SLC Japan (Shizuoka, Japan) and fed either GGOH supplemented (48.3 mg/kg of diet) or control diet for 10 days. Growth performance did not differ between both diets, but plasma testosterone levels in GGOH supplemented group were found to be elevated compared to that of control group, indicating that dietary supplementation of GGOH significantly elevates plasma testosterone levels (unpublished data, Figure ). These findings provide novel mechanistic insights into the process of testosterone production and may be useful for the development of therapeutic strategies to counter age-associated declines in testosterone levels in men.
Figure 4. Dietary supplementation of GGOH enhances plasma testosterone levels in male Wistar rats. Data are presented as mean ± SD (n = 7 − 8). *p < 0.05 vs. Ctrl group.
In summary, the novel role of GGOH in steroidogenesis may bring new possibilities and could be useful in the development of therapeutics for the treatment of men with LOH.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was partially supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) to HS [grant number #17H03814], and by the JSPS Core-to-Core Program A (Advanced Research Networks) entitled: “Establishment of international agricultural immunology research-core for a quantum improvement in food safety.”
Acknowledgement
We are deeply grateful to Tama Biochemical Co. Ltd. (Tokyo, Japan) for providing all trans-GGOH used in animal experiments.
References
- Christianson DW. Unearthing the roots of the terpenome. Curr Opin Chem Biol. 2008;12:141–150.10.1016/j.cbpa.2007.12.008
- Ahlquist L, Bergström G, Liljenberg C. Acyclic diterpene alcohols: occurrence and synthesis of geranylcitronellol, phytol and geranylgeraniol. Prog Chem Fats Other Lipids. 1978;16:231–255.10.1016/0079-6832(78)90046-0
- Chappell J. Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu Rev Plant Phys. 1995;46:521–547.10.1146/annurev.pp.46.060195.002513
- Bach TJ, Rohmer M. Isoprenoid synthesis in plants and microorganisms. New York (NY): Springer; 2012. p. 505.
- Cragg GM. Paclitaxel (Taxol): a success story with valuable lessons for natural product drug discovery and development. Med Res Rev. 1998;18:315–331.10.1002/(ISSN)1098-1128
- Dhingra V, Rao KV, Narasu ML. Current status of artemisinin and its derivatives as antimalarial drugs. Life Sci. 2000;66:279–300.
- Lange BM, Rujan T, Martin W, et al. Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Nat Acad Sci USA. 2000;97:13172–13177.10.1073/pnas.240454797
- Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3:408–414.10.1038/nchembio.2007.5
- Muraguchi T, Okamoto K, Mitake M, et al. Polished rice as natural sources of cancer-preventing geranylgeranoic acid. J Clin Biochem Nutr. 2011;49:8–15.10.3164/jcbn.10-110
- Fisher JE, Rogers MJ, Halasy JM, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Nat Acad Sci USA. 1999;96:133–138.10.1073/pnas.96.1.133
- Benford HL, Frith JC, Auriola S, et al. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol Pharmacol. 1999;56:131–140.
- Van Beek ER, Löwik CW, Papapoulos SE. Bisphosphonates suppress bone resorption by a direct effect on early osteoclast precursors without affecting the osteoclastogenic capacity of osteogenic cells: the role of protein geranylgeranylation in the action of nitrogen-containing bisphosphonates on osteoclast precursors. Bone. 2002;30:64–70.10.1016/S8756-3282(01)00655-X
- Riebeling C, Forsea AM, Raisova M, et al. The bisphosphonate pamidronate induces apoptosis in human melanoma cells in vitro. Br J Cancer. 2002;87:366–371.10.1038/sj.bjc.6600476
- Hiruma Y, Nakahama K, Fujita H, et al. Vitamin K2 and geranylgeraniol, its side chain component, inhibited osteoclast formation in a different manner. Biochem Biophys Res Commun. 2004;314:24–30.10.1016/j.bbrc.2003.12.051
- Ziebart T, Koch F, Klein MO, et al. Geranylgeraniol – a new potential therapeutic approach to bisphosphonate associated osteonecrosis of the jaw. Oral Oncol. 2011;47:195–201. doi:10.1016/j.oraloncology.2010.12.003.
- Fisher JE, Rosenberg E, Santora AC, et al. In vitro and in vivo responses to high and low doses of nitrogen-containing bisphosphonates suggest engagement of different mechanisms for inhibition of osteoclastic bone resorption. Calcif Tissue Int. 2013;92:531–538.10.1007/s00223-013-9711-0
- Pabst AM, Krüger M, Ziebart T, et al. Isoprenoid geranylgeraniol: the influence on cell characteristics of endothelial progenitor cells after bisphosphonate therapy in vitro. Clin Oral Investig. 2015;19:1625–1633.10.1007/s00784-014-1394-z
- Frenkel J, Rijkers GT, Mandey SH, et al. Lack of isoprenoid products raises ex vivo interleukin-1beta secretion in hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis Rheum. 2002;46:2794–2803.10.1002/(ISSN)1529-0131
- Marcuzzi A, Pontillo A, Leo L, et al. Natural isoprenoids are able to reduce inflammation in a mouse model of mevalonate kinase deficiency. Pediatr Res. 2008;64:177–182.10.1203/PDR.0b013e3181761870
- Ownby SE, Hohl RJ. Farnesol and geranylgeraniol: prevention and reversion of lovastatin-induced effects in NIH3T3 cells. Lipids. 2002;37:185–192.10.1007/s11745-002-0879-1
- Campia I, Lussiana C, Pescarmona G, et al. Geranylgeraniol prevents the cytotoxic effects of mevastatin in THP-1 cells, without decreasing the beneficial effects on cholesterol synthesis. Br J Pharmacol. 2009;158:1777–1786.10.1111/j.1476-5381.2009.00465.x
- Kim J, Lee JN, Ye J, et al. Sufficient production of geranylgeraniol is required to maintain endotoxin tolerance in macrophages. J. Lipid Res. 2013;54:3430–3437.10.1194/jlr.M042549
- Inoue Y, Hada T, Shiraishi A, et al. Biphasic effects of geranylgeraniol, teprenone, and phytol on the growth of Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:1770–1774.10.1128/AAC.49.5.1770-1774.2005
- Montero MT, Matilla J, Gomez-Mampaso E, et al. Geranylgeraniol regulates negatively caspase-1 autoprocessing: implication in the Th1 response against Mycobacterium tuberculosis. J. Immunol. 2004;173:4936–4944.10.4049/jimmunol.173.8.4936
- Giriwono PE, Shirakawa H, Ohsaki Y, et al. Dietary supplementation with geranylgeraniol suppresses lipopolysaccharide-induced inflammation via inhibition of nuclear factor-κB activation in rats. Eur J Nutr. 2013;52:1191–1199.10.1007/s00394-012-0429-y
- Masuda Y, Maeda S, Watanabe A, et al. A novel 21-kDa cytochrome c-releasing factor is generated upon treatment of human leukemia U937 cells with geranylgeraniol. Biochem Biophys Res Commun. 2006;346:454–460.10.1016/j.bbrc.2006.05.161
- Fernandes NV, Yeganehjoo H, Katuru R, et al. Geranylgeraniol suppresses the viability of human DU145 prostate carcinoma cells and the level of HMG CoA reductase. Exp Biol Med. 2013;238:1265–1274.
- Ohizumi H, Masuda Y, Nakajo S, et al. Geranylgeraniol is a potent inducer of apoptosis in tumor cells. J Biochem. 1995;117:11–13.10.1093/oxfordjournals.jbchem.a124695
- Yeganehjoo H, DeBose-Boyd R, McFarlin BK, et al. Synergistic impact of d -δ-tocotrienol and geranylgeraniol on the growth and HMG CoA reductase of human DU145 prostate carcinoma cells. Nutr Cancer. 2017;69:682–691.10.1080/01635581.2017.1299876
- Mailman T, Hariharan M, Karten B. Inhibition of neuronal cholesterol biosynthesis with lovastatin leads to impaired synaptic vesicle release even in the presence of lipoproteins or geranylgeraniol. J Neurochem. 2011;119:1002–1015.10.1111/jnc.2011.119.issue-5
- Marcuzzi A, Piscianz E, Zweyer M, et al. Geranylgeraniol and neurological impairment: involvement of apoptosis and mitochondrial morphology. Int J Mol Sci. 2016;17:365.10.3390/ijms17030365
- Ho HJ, Shirakawa H, Yoshida R, et al. Geranylgeraniol enhances testosterone production via the cAMP/protein kinase A pathway in testis-derived I-10 tumor cells. Biosci Biotechnol Biochem. 2016;80:691–697.
- Sriraman V, Anbalagan M, Rao AJ. Hormonal regulation of Leydig cell proliferation and differentiation in rodent testis: a dynamic interplay between gonadotrophins and testicular factors. Reprod Biomed Online. 2005;11:507–518.10.1016/S1472-6483(10)61147-9
- Payne AH, Hardy MP. The Leydig cell in health and disease. Totowa (NJ): Human Press; 2007.10.1007/978-1-59745-453-7
- Shores MM, Matsumoto AM, Sloan KL, et al. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–1665.10.1001/archinte.166.15.1660
- Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202.10.4103/1008-682X.122336
- Dudek P, Kozakowski J, Zgliczyński W. Late-onset hypogonadism. Prz Menopauzalny. 2017;16:66–69.
- Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210:232–236.10.1016/j.atherosclerosis.2009.10.037
- Morris PD, Channer KS. Testosterone and cardiovascular disease in men. Asian J Androl. 2012;14:428–435.10.1038/aja.2012.21
- Beatrice AM, Dutta D, Kumar M, et al. Testosterone levels and type 2 diabetes in men: current knowledge and clinical implications. Diabetes Metab Syndr Obes. 2014;7:481–486.
- Yeap BB. Testosterone and cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2015;22:193–202.10.1097/MED.0000000000000161
- Caminiti G, Volterrani M, Iellamo F, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a doubleblind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–927.10.1016/j.jacc.2009.04.078
- Saad F, Aversa A, Isidori AM, et al. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011;165:675–685.10.1530/EJE-11-0221
- Araujo AB, Dixon JM, Suarez EA, et al. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–3019.10.1210/jc.2011-1137
- Ito A, Shirakawa H, Takumi N, et al. Menaquinone-4 enhances testosterone production in rats and testis-derived tumor cells. Lipids Health Dis. 2011;10:158.10.1186/1476-511X-10-158
- Ohsaki Y, Shirakawa H, Miura A, et al. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J Nutr Biochem. 2010;21:1120–1126.10.1016/j.jnutbio.2009.09.011
- Holstein SA, Hohl RJ. Isoprenoids: remarkable diversity of form and function. Lipids. 2004;39:293–309.10.1007/s11745-004-1233-3
- Joo JH, Jetten AM. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 2010;287:123–135.10.1016/j.canlet.2009.05.015
- Chowdhury RR, Ghosh SK. Phytol-derived novel isoprenoid immunostimulants. Front Immunol. 2012;3:49.
- Gancedo JM. Biological roles of cAMP: variations on a theme in the different kingdoms of life. Biol Rev Camb Philos Soc. 2013;88:645–668.10.1111/brv.2013.88.issue-3
- Steegborn C. Structure, mechanism, and regulation of soluble adenylyl cyclases-similarities and differences to transmembrane adenylyl cyclases. Biochim Biophys Acta. 2014;1842:2535–2547.10.1016/j.bbadis.2014.08.012
- Hacker BM, Tomlinson JE, Wayman GA, et al. Cloning, chromosomal mapping, and regulatory properties of the human type 9 adenylyl cyclase (ADCY9). Genomics. 1998;50:97–104.10.1006/geno.1998.5293
- Stocco DM, Wang X, Jo Y, et al. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19:2647–2659.10.1210/me.2004-0532
